2 Principles and steps T 22
Quality management principles, preparation and implementation steps, Deming cycle
Quality is anything that can be improved. Masaaki Imai
The qualityaptitude to fulfill requirements (see also ISO 9000, 3.6.2) approach is a state of mind which starts with top management as a priority strategic decision and extends to all employees. Top managementgroup or persons in charge of the organizational control at the highest level (see also ISO 9000, 3.1.1) develops a quality policystatement by top management allowing the establishment of quality objectives (see also ISO 9000, 3.5.9) which determines the quality objectivesquality-related, measurable goal that must be achieved (see also ISO 9000, 3.7.2), themselves applicable to all activities. The tool used to achieve the objectives is the quality system. Prevention is a key concept of quality management systemsset of processes allowing the achievement of the quality objectives (see also ISO 9000, 3.5.4).
Qualityaptitude to fulfill requirements (see also ISO 9000, 3.6.2) is almost free when customersanyone who receives a product (see also ISO 9000, 3.2.4) are satisfied: they remain loyal to us. It’s only when the customeranyone who receives a product (see also ISO 9000, 3.2.4) is not fully satisfied that qualityaptitude to fulfill requirements (see also ISO 9000, 3.6.2) becomes very expensive to us: sooner or later the customeranyone who receives a product (see also ISO 9000, 3.2.4) will go to a competitor.
Quality stays long after the price has been forgotten
The seven quality managementactivities allowing the control of an organization with regard to quality (see also ISO 9000, 3.3.4) principles (cf. figure 2-1) will help us achieve sustained success (cf. ISO 9000: 2015, sub-clause 2.3).
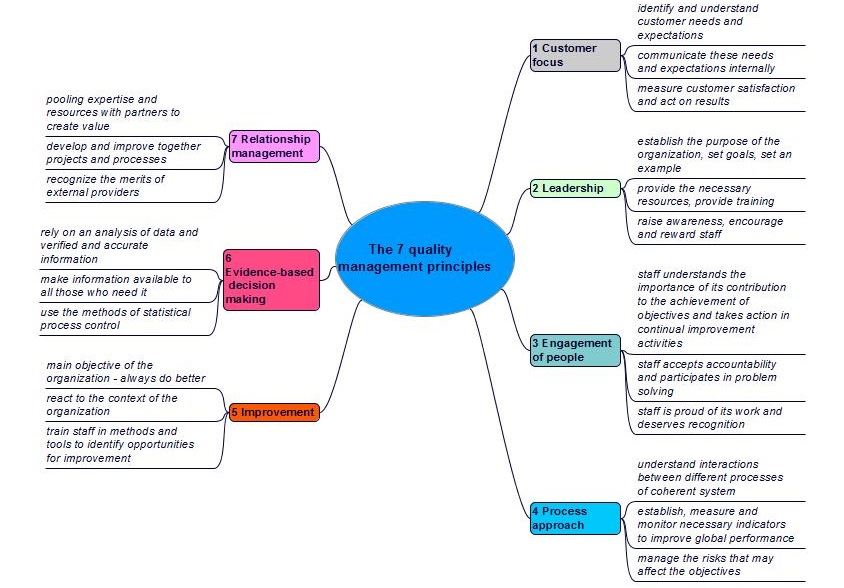
Figure 2-1. The 7 quality management principles
A well-prepared approach is halfway to success
The approach to implementing a quality management systemset of processes allowing the achievement of the quality objectives (see also ISO 9000, 3.5.4) (QMSQuality Management System) starts with preparation. An example is shown in figure 2-2.
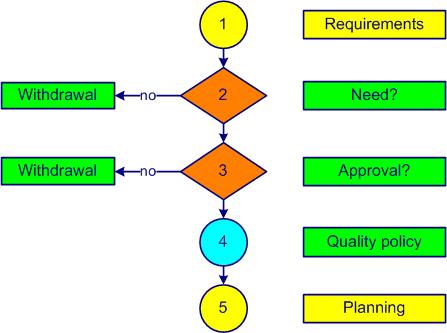
Figure 1-4. IMS preparation
Step one involves identifying the needs and expectations (requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.6.4)) of customersanyone who receives a product (see also ISO 9000, 3.2.4) (internal and external). The involvement of top managementgroup or persons in charge of the organizational control at the highest level (see also ISO 9000, 3.1.1) at its highest level is truly indispensable. The advice of a consultant is often solicited. An external certification body is chosen.
One of the key questions that comes up quickly (step 2) is the need for this decision. If this is not really necessary or if the estimated costs of the certification approach exceed the available resources, it is better to dismiss the idea immediately.
The ISO 9000 family of standards will stop you making promises you can't fulfil and help you keep those you can. David Hoyle
The benefits of implementing a quality management system are often:
- an improved image of the organization
- being one step ahead of competition
- better economic results
- increased daily effectiveness
- staff who are aware, consulted, motivated and proud
- best practices are valorized
- formalization of knowledge
- process control
- applicable regulatory requirements
- reduced production costs
- profitable engagement for all
The benefits of the certification of a quality management system are often:
- new customers
- an increased market share
- increase in sales
- better financial performance
More than one and a half million businesses worldwide can not be wrong!
Medtronic, a global leader in medical technology, has implemented ISO 13485 to ensure the quality of its products and improve patient safety. The standardization of procedures and the establishment of a quality culture have made it possible to reduce manufacturing defects and improve the reliability of medical products.
Medtronic has seen significant improvement in product quality, demonstrating the company's commitment to patient safety and the positive impact of ISO 13485 on financial performance
The internalization of the spirit of the principles and requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.6.4) of an ISO standard significantly improves the overall performancemeasurable and expected results of the management system (see also ISO 9000, 3.7.8) of your business, especially when it is not considered as a constraint.
The third step shall determine whether this approach receives the approval of the staff. A communicationexchange of information campaign is launched in-house on the objectives of a quality management systemset of processes allowing the achievement of the quality objectives (see also ISO 9000, 3.5.4) (QMSQuality Management System). The staff is aware and understands that, without their participation, the project cannot succeed.
Have confidence: success will come with the involvement and effort of all!
The vision (what we want to be), the mission (why we exist) and the business plan of the company are set. The next step (4) includes the addition in the quality policy of the specific regulatory requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.6.4) of medical devicesproduct or service to be used for purposes of diagnosis, prevention, monitoring, treatment, alleviation of disease or injury. If you do not have a copy of the ISO 13485 standard, now is the time to get it.
Planning is the last step (5) of the project preparation for obtaining ISO 13485 certification. A reasonable period is between 5 to 8 months (each company is unique and specific). The financial resources and staff are confirmed by top managementgroup or persons in charge of the organizational control at the highest level (see also ISO 9000, 3.1.1). A management representative (often it is the quality managerleader of the journey towards excellence) is appointed as project leader. Top managementgroup or persons in charge of the organizational control at the highest level (see also ISO 9000, 3.1.1) commitment is formalized in a document communicated to all staff. A person is appointed project leader for obtaining certification.
The establishment and implementation of a QMSQuality Management System are shown in figure 2-3.
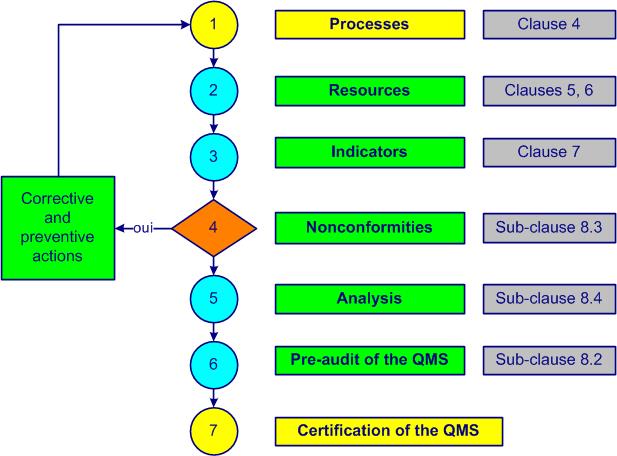
Figure 2-3. QMS implementation
Step 1 aims to identify and determine the processesactivities that transform inputs into outputs (see also ISO 9000, 3.4.1) and establish specific requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.6.4) in processesactivities that transform inputs into outputs (see also ISO 9000, 3.4.1) and documentsany support allowing the treatment of information (see also ISO 9000, 3.8.5).New documentsany support allowing the treatment of information (see also ISO 9000, 3.8.5) are created. The quality manualdocument specifying the general measures taken by an organization to obtain conforming products or services (see also ISO 9000, 3.8.8) is updated.
In step 2 the necessary resources to achieve the quality objectivesquality-related, measurable goal that must be achieved (see also ISO 9000, 3.7.2) are determined. Planning tasks, responsibilities and time frames are established. Training of internal auditorseveryone who is trained to carry out audits (see also ISO 19011, 3.8) is taken into account.
Step 3 allows you to set and implement methods for measuring the effectivenesscapacity to perform planned activities with minimum effort (see also ISO 9000, 3.7.11) and efficiencyfinancial relationship between achieved results and resources used (see also ISO 9000, 3.7.10) of each processactivities that transform inputs into outputs (see also ISO 9000, 3.4.1) (indicators). Internal auditssystematic and independent survey to determine whether activities and results comply with pre-established measures and are capable of achieving the objectives (see also ISO 19011, 3.1) help to evaluate the degree of implementation of specific requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.6.4).
Nonconformitiesnon-fulfillment of a specified requirement (see also ISO 9000, 3.6.9) of all kinds are listed in step 4. A first draft for dealing with wasteanything that adds cost but no value is established. Corrective andpreventive actionsaction to eliminate the potential causes of nonconformity or any other undesirable event and to prevent their appearance (see also ISO 9000, 3.6.4 and ISO 14 001, 3.17) are implemented and documented. An approach to preventing nonconformitiesnon-fulfillment of a specified requirement (see also ISO 9000, 3.6.9) and eliminating causes is established.
A first encounter with analysis of data is made in step 5. A table with the main costs of obtaining quality (COQcosts of obtaining quality) is filled in by those with the information at hand. Feedback from customersanyone who receives a product (see also ISO 9000, 3.2.4) and other data is analyzed to evaluate the adequacy and effectivenesscapacity to perform planned activities with minimum effort (see also ISO 9000, 3.7.11) of the QMSQuality Management System.
To conduct the pre-audit of the QMSQuality Management System (step 6), documentation such as quality manualdocument specifying the general measures taken by an organization to obtain conforming products or services (see also ISO 9000, 3.8.8), proceduresdocument describing the to carry out a process (see also ISO 9000, 3.4.5 and documented information) and others are checked and approved by the appropriate people. A management reviewperiodic survey carried out by top management of the management system for its continual improvement allows evaluation of compliance with applicable regulatory requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.6.4). The quality policystatement by top management allowing the establishment of quality objectives (see also ISO 9000, 3.5.9) and objectives are finalized. A quality managerleader of the journey towards excellence from another company or a consultant can provide valuable feedback, suggestions and recommendations.
When the systemset of interacting processes (see also ISO 9000, 3.5.1) is accurately implemented and followed, the certification of the QMSQuality Management System by an external body is a breeze, a formality (step 7).
An example of a certification project plan is shown in annex 01. 
An appropriate method for evaluating the performancemeasurable and expected results of the management system (see also ISO 9000, 3.7.8) of your integrated management systemset of processes to achieve QSE objectives is the RADAR logic model of excellence EFQM (European Foundation for Quality Management) with its nine criteria and overall score of 1000 points.
The Deming cycle (figure 2-4) is applied to control any processactivities that transform inputs into outputs (see also ISO 9000, 3.4.1). The PDCA cycles (Plan, Do, Check, Act) are a universal base for continual improvementprocess allowing the improvement of the global performance of the organization (see also ISO 9000, 3.3.2).
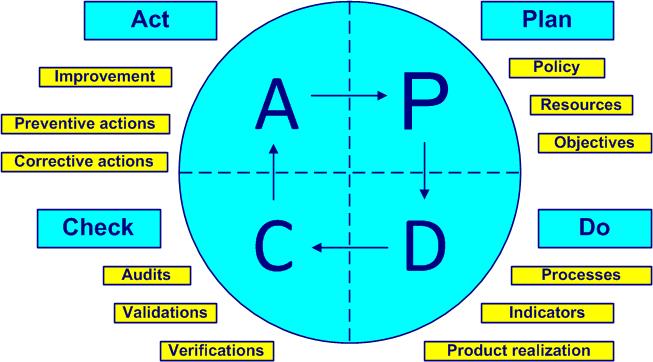
Figure 2-4. Deming cycle
- Plan – define and establish strategy, customers, policy, resources, objectives,documentation, products, processes, training and deadlines (ISO 13485, clauses 4, 5 and 6)
- Do – implement processes, indicators and product realization (ISO 13485 clause 7)
- Check – inspect, analyze data, verify if objectives are achieved, validate and audit (ISO 13485 clause 8)
- Act – adapt, adjust, improve, react with actions or find new improvements (new PDCA cycle), (ISO 13485 sub-clauses 5.6, 8.3 and 5.5)
For more information on the Deming cycle and its 14 points of management theory, you can consult the classic book "Out of the Crisis", W. Edwards Deming, MIT press, 1982.
