1 Quality approach
1.1 Background
Quality standards' evolution
The evolution of the quality concept and the standards of quality management systems (Quality Management System = QMS) in industrial countries in the 20th century can be summarized as:
- quality control (till the 1980s) – quality practices, customers are (or seem) satisfied
- quality assurance (the 1990s) – the system is determined and implemented
- quality management (ISO 9000 : 2000) – the system is controlled and its efficiency is improved
The technical committee "Management and quality assurance" (ISO/TC 176) within the ISO (International Organization for Standardization) was created in 1980. ISO itself was created in 1947. ISO comes from the Greek "isos" (equal).
The ISO 9000 standards (see figure 1-1) have appeared in:
- 1987: ISO 9000 first edition, based on the US military standard MIL-Q-9858 of 1959
- 1994: ISO 9000 revision n° 1: ISO 9001; ISO 9002; ISO 9003; ISO 9004 – more understandable, customer focus better determined, preventive actions added
- 2000: ISO 9000 revision n° 2: ISO 9000; ISO 9001; ISO 9004 – simplified structure (8 clauses), priority to process approach and customer satisfaction
- 2008: third revision (fourth edition of ISO 9001): clarification of the requirements (no new requirement), better alignment with ISO 14 001
- 2015: fourth revision, new structure (high level), added risk-based thinking, performance becomes a priority, lightweight documentation
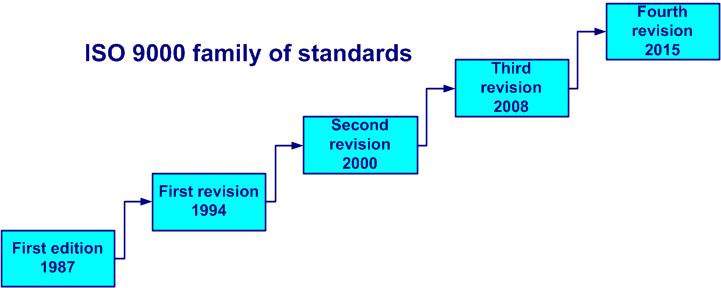
Figure 1-1. Revisions of ISO 9000 family
The fourth edition (revision, version) of ISO 9001 was published in 2015.
The standard “ISO 19443 - Quality management systems — Specific requirements for the application of ISO 9001:2015 by organizations in the supply chain of the nuclear energy sector supplying products and services important to nuclear safety (ITNS)” was published in 2018.
Some historical points (standards and references) related with the creation of the ISO 19443 standard, of French initiative, are shown in figure 1-2:
Figure 1-2. History of ISO 19443
Atomic Energy Act, US Congress, 1946
N45.2 Quality Assurance Program Requirements for Nuclear Facilities, ANSI, 1971
GS-R-3 IAEA, The management system for facilities and activities, Safety requirements, IAEA, 2006
NQA – 1, Nuclear Quality Assurance - Quality Assurance Requirements for Nuclear Facility Applications, ASME, 2008
NSQ-100, Nuclear Safety and Quality Management System Requirements, NQSA, 2010
DOE O 450.2, Integrated Safety Management, US Department of Energy, 2011
Decree of 7 February 2012, Order of February 7, 2012 establishing the general rules relating to basic nuclear installations (BNI), French laws, 2012
GSR Part 2, General Safety Requirements for Leadership and Management for Safety, IAEA, 2016
AS9100 - Quality Management Systems – Requirements for Aviation, Space and Defense Organizations, IAQG, 2016
The requirements of ISO 19443 are not intended to replace customer, statutory and regulatory requirements, but are complementary.
In addition to ISO 9001, the ISO 19443 requirements mainly focus on:
- issues that include nuclear safety considerations
- nuclear safety is taken into account in decision making
- nuclear safety culture
- ITNS items and activities break down
- graded approach to the application of quality requirements
- changes to the QMS are managed so nuclear safety is not compromised
- resources are provided so nuclear safety is not compromised
- competence of persons also address specific qualification
- provisions for counterfeit, fraudulent or suspect items (CFSI)
- project and configuration management
- independence of verification and validation of design and development
- design and development verification and validation testing
- evaluation whether external providers meet the requirements of ISO 19443
- ITNS purchasing requirements to all levels of the supply chain
- enhanced traceability
- control of production equipment
- monitoring and measurement activities
- preservation of important to nuclear safety (ITNS) products
- statement of conformity at delivery
- root cause analysis of nonconformities
- analysis and evaluation of nuclear safety culture aspects
- relations with nuclear safety authorities
- nuclear safety culture is included in continual improvement opportunities
1.2 Scope
Scope of the QMS, when certain requirements cannot be applied
The ISO 9001 standard (Quality management systems - Requirements) is generic as it can be applied to the management system of any company, without limitations on size, activity or type. It is a voluntary international standard which allows certification by accredited bodies.
The scope of ISO 19443 applies to any organization supplying ITNS (important to nuclear safety) products or services.
Nevertheless, certain requirements cannot be applied in particular cases. This is possible when:
- it does not affect product and service conformity and nuclear safety in any way
- it does not relieve top management of its responsibilities
- it is justified in a document
1.3 Principles and steps
Quality management principles, preparation and implementation, Deming cycle
Quality is anything that can be improved. Masaaki Imai
The quality approach is a state of mind which starts with top management as a priority strategic decision and extends to all employees. Top management develops a quality policy which determines the quality objectives, themselves applicable to all activities. The tool used to achieve the objectives is the quality system. Prevention is a key concept of quality management systems.
Quality management systems include three distinct and interrelated steps:
- process approach
- risk approach
- continual improvement
The purpose of a quality management system is to increase the satisfaction of customers (both external and internal) by meeting their needs and expectations through continual improvement of the effectiveness of the processes.
Quality is almost free when customers are satisfied: they remain loyal to us. It’s only when the customer is not fully satisfied that quality becomes very expensive to us: sooner or later the customer will go to a competitor.
Quality remains long after the price has been forgotten
The seven quality management principles (cf. figure 1-3) will help us achieve sustained success (cf. ISO 9000: 2015, sub-clause 2.3). Previously there were eight but now the system approach is integrated into the process approach.
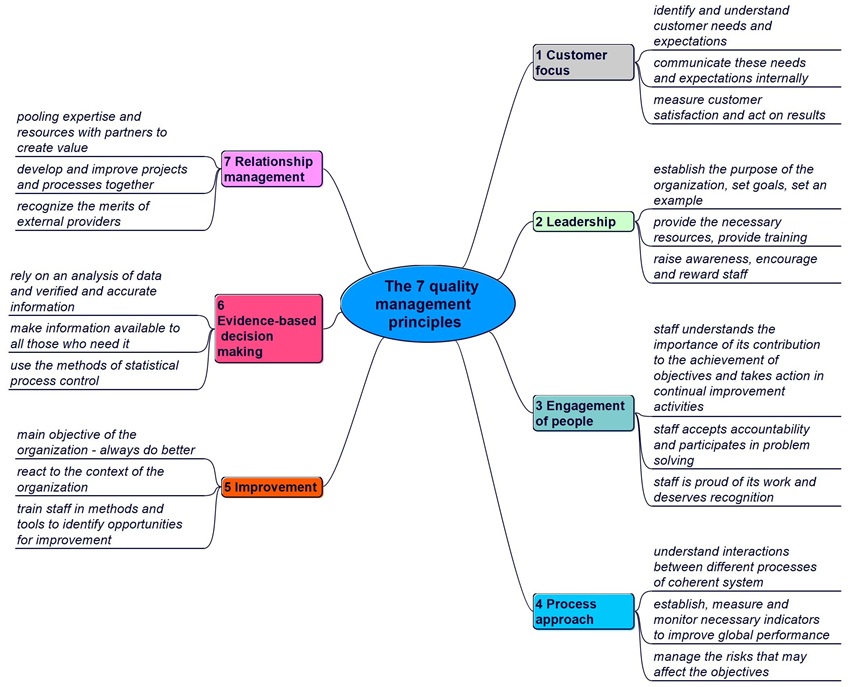
Figure 1-3. The 7 quality management principles
A well-prepared approach is half successful
The approach to implementing a nuclear safety management system starts with the preparation. An example is shown in figure 1-4.
.gif)
Figure 1-4. NSMS preparation
Step 1 involves identifying the needs and expectations (requirements) of stakeholders
- staff
- customers, consumers
- competitors
- stakeholders, investors
- external providers (suppliers, subcontractors, partners)
- organizations and branch associations
- statutory and regulatory authorities
The involvement of top management at its highest level is truly indispensable. The advice of a consultant is often solicited. Determining the current status of the management system (whole or partial) would be welcome at this stage. An external certification body is chosen.
One of the key questions that comes up quickly (step 2) is the need for this decision. If this is not really necessary or if the estimated costs of the certification approach exceed the available resources, it is better to reject this idea immediately.
The ISO 9000 family of standards will stop you making promises you can't fulfil and help you keep those you can. David Hoyle
The benefits of implementing a management system are often:
- an improved image of the organization
- being one step ahead of the competition
- enhanced customer satisfaction
- better economic results
- increased daily efficiency
- staff is aware, consulted, motivated and proud
- high level of risk control
- reduced insurance costs
- profitable engagement for all
- best practices valorized
- formalization of knowledge
- process control
The benefits of ISO 19443 certification are often:
- enhanced safety and quality:
- nuclear-specific requirements (a NSMS that actively "designs safety in")
- reduced risks and failures:
- systematic identification and mitigation of risks throughout the supply chain (it minimizes the potential for defects, failures, and non-conformities)
- increased credibility and trust:
- demonstrated commitment (clear, independent proof of a company's dedication to the highest standards of quality and nuclear safety)
- stronger stakeholder confidence:
- it builds trust with regulators, customers (nuclear plant operators), and other stakeholders, fostering stronger, long-lasting partnerships
- global recognition:
- it facilitates acceptance by regulatory bodies worldwide, streamlining processes for international projects
- competitive advantage and market access:
- entry to the nuclear supply chain (ISO 19443 is increasingly becoming a prerequisite and a significant differentiator)
- simplifies supplier qualification:
- nuclear operators often have strict vendor qualification processes (this accreditation signals reliability and quality)
- standardization:
- it helps standardize quality requirements across the global nuclear supply chain (reducing inconsistencies and complexities)
- operational efficiency and continual improvement:
- streamlined processes (the standard promotes a structured, process-oriented approach, which can lead to optimized workflows and reduced waste)
- culture of excellence:
- it embeds a nuclear safety culture and a mindset of continual improvement
- regulatory compliance:
- meets stringent demands (ISO 19443 helps companies meet complex national and international nuclear safety regulations)
- traceability and counterfeit prevention:
- it emphasizes enhanced traceability requirements and includes measures to counteract counterfeit, fraudulent, and suspect items (CFSIs)
Mirion Technologies (France & Germany), a supplier of radiation measurement solutions, sought to ensure maximum nuclear safety in a highly sensitive field.
Several Mirion sites obtained ISO 19443 accreditation (certification). The audits highlighted effective collaboration between their departments and their methodical approach to projects.
The benefit: the accreditation validates the robustness of their quality management system, their unwavering commitment to nuclear safety and their ability to provide reliable products and services for the nuclear environment.
The internalization of the spirit of the principles and requirements of an ISO standard significantly improves the overall performance of your business, especially when it is not considered as a constraint.
The third step shall determine whether this approach receives the approval of the staff. A communication campaign is launched in-house on the objectives of a nuclear safety management system (NSMS). The staff is aware and understands that, without their participation, the project cannot succeed.
Have confidence: success will come with the involvement and effort of all!
The vision (what we want to be), the mission (why we exist) and the business plan of the company are determined. The following step (4) includes the establishment of an outline of the quality policy and quality objectives. If you do not have a copy of the ISO 19443 standard, now is the time to get it (see sub-clause 2.1 of the present course).
Planning is the last step (5) of the project preparation for obtaining ISO 19443 certification. A reasonable period is between 5 to 8 months (each company is unique and specific). The financial resources and staff are confirmed by top management. A management representative is appointed as project leader. Top management commitment is formalized in a document communicated to all staff. A person is appointed project leader for obtaining ISO 19443 certification.
The establishment and implementation of an ISO 19443 nuclear safety management system are shown in figure 1-5.
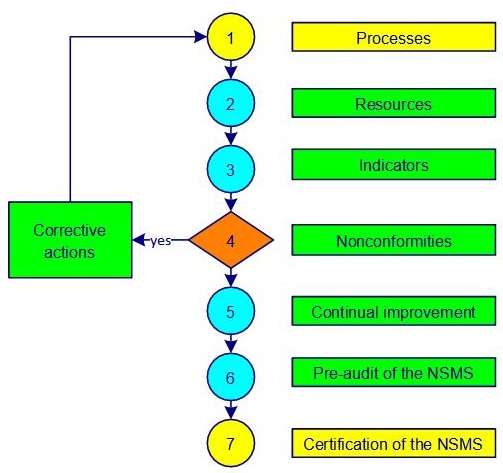
Figure 1-5. NSMS implementation
Step 1 aims to identify and determine the processes, interactions, owners, responsibilities and drafts of certain documents. The first versions of process sheets, job descriptions and work instructions are written with the participation of the maximum number of available persons.
The necessary resources to achieve the quality objectives are determined in step 2. Planning tasks, responsibilities and time frames are established. Training of internal auditors is taken into account.
Step 3 allows you to set and implement methods for measuring the effectiveness and efficiency of each process (indicators). Internal audits help to evaluate the degree of implementation of the system.
Nonconformities of all kinds are listed in step 4. A first draft for dealing with waste is established. Corrective actions are implemented and documented. A sorting out of correctve actions is introduced.
A first encounter with the tools and application areas of continual improvement is made in step 5. A table with the main costs of obtaining quality (COQ) is filled by people with the information at hand. Risks are determined, actions are planned and improvement opportunities are found. An approach to preventing nonconformities and eliminating causes is established. The internal and external communication is established and formalized.
To conduct the pre-audit of the NSMS (step 6), documentation is checked and approved by the appropriate people. A management review allows evaluation of compliance with applicable requirements. The quality policy and objectives are finalized. A quality manager from another company or a consultant can provide valuable feedback, suggestions and recommendations.
When the system is accurately implemented and followed, the certification of the NSMS is a breeze, a formality (step 7).
An example of a certification project plan with 26 steps is shown in annex 01. 
An appropriate method for evaluating the performance of your nuclear safety management system is the RADAR logic model of excellence EFQM (European Foundation for Quality Management) with its nine criteria and overall score of 1000 points.
The Deming cycle (figure 1-5) is applied to control any process. The PDCA cycles (Plan, Do, Check, Act) are a universal base for continual improvement.
- Plan – define context, issues, and processes, demonstrate leadership, establish quality policy and objectives (clauses 4, 5 and 6)
- Do – realize the product, develop, implement and control processes, demonstrate leadership, bring support (clauses 5, 7 and 8)
- Check – compare, evaluate, inspect, analyze data, conduct audits and management reviews, demonstrate leadership (clauses 5 and 9)
- Act – adapt, demonstrate leadership, treat nonconformities, react with corrective actions and find new improvements (new PDCA cycle), (clauses 5 and 10)
For more information on the Deming cycle and his 14 points of management theory you can consult the classic book "Out of the crisis", W. Edwards Deming, MIT press, 1982.

.jpg)