1 Good Manufacturing Practices
- 1.1 History
- 1.2 Benefits
- 1.3 Application
- 1.4 Standards, definitions and books
- 1.5 Principles and steps
1.1 History
Standards and cosmetic regulations
Other cosmetic proverbs and quotes
A "Partial Agreement" in the social and public health field was concluded in 1959 by seven Council of Europe member states (Belgium, France, Germany, Italy, Luxembourg, the Netherlands and the United Kingdom). This "Partial Agreement" was binding only on the signatory states. Subsequently many other states joined the Partial Agreement.
In 1976 the Council Directive 76/768/EEC on the “rapprochement” of the laws of the Member States relating to cosmetic products was published.
In 1994 COLIPA, (now Cosmetics Europe) the European Cosmetics and Perfume Association published the Good Manufacturing Practices for Cosmetics.
In 1995 the Council of Europe published the "Guidelines on Good Production Practice for Cosmetic Products (GPPC), Consumer Health Protection", the result of a study commissioned by the Committee of Experts on Cosmetic Products. These recommendations, intended to guide cosmetics production companies, concern the different stages of the production processactivities which transform inputs into outputs (see also ISO 9000, 3.4.1) allowing the control of factors affecting productany outcome of a process or activity (see also ISO 9000, 3.4.2) quality.
In 2003 ASEAN (Association of Southeast Asian Nations) published an agreement on the Cosmetic Regulatory Scheme.
In 2006 the European Commission amended Decision 96/335/EC on the establishment of an inventory and a common nomenclature of ingredients employed in cosmetic products, Decision 2006/257/EC.
In 2007 the ISOinternational organization for standardization (International Organization for Standardization) publishes the international standard ISO 22716 "Guidelines on Good Manufacturing Practices, Cosmetics". These are guidelines intended to ensure the manufacturing quality of cosmetic products concerning the different processes of production, control, storage and shipment. In form, the document is a standard, but in substance, the text is a guideline (use of the verb "should" 238 times). Only the aspects related to the quality and safety of the productany outcome of a process or activity (see also ISO 9000, 3.4.2) are taken into account. These guidelines do not cover aspects related to productany outcome of a process or activity (see also ISO 9000, 3.4.2) design, personnel safety or environmental protection.
Regulation (EC) No 1272/2008, known as CLP (Classification, Labeling and Packaging) of the European Parliament and of the Council of 2008, concerns the classification, labeling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC and amending Regulation (EC) No 1907/2006. In France it has been in force since 11 July 2013.
The "Cosmetics Regulation" (EC) 1223/2009 of the European Parliament and of the Council of 30 November 2009, which entered into force on 11 July 2013, relating to cosmetic products is an essential text for any cosmetics producer (replaces Directive 76/768/EC). The Cosmetic Regulation requires that each cosmetic product placed on the European market has been manufactured in accordance with the Good Manufacturing Practices described in the ISO 22716 standard.
Commission Regulation (EU) No. 655/2013 of 10 July 2013 (the so-called "Claims Regulation") establishes common criteria that cosmetic product claims must meet in order to be used.
The implementing Commission decision of 25 November 2013 2013/674/EU concerns the Guidelines for the application of Annex I to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products.
In 2013 the FDA (Food and Drug Administration) publishes recommendations under the title Cosmetic Good Manufacturing Practices - Draft Guidance based on the ISO 22716 standard.
Law No. 2014-201 of 24 February 2014 includes the various provisions for adaptation to European Union law in the field of health in French. One part concerns cosmetic products and the amendments in the articles of the Public Health Code.
Commission Regulation (EU) 2015/830 of 28 May 2015 amends Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) and contains in its Annex II the requirements for drawing up the Safety Data Sheet (SDS).
In 2015, the Institute for Reference Materials and Measurements will publish the Guidelines for Analytical Methods in Cosmetics (for use by laboratories) - JRC guidelines for selecting and/or validating analytical methods for cosmetics, and recommending standardization steps for analytical methods for cosmetics.
Decree No. 2015-1417 of 2015, in French, relates to cosmetic products and tattoo productsany outcome of a process or activity (see also ISO 9000, 3.4.2).
The decree of 30 November 2016 sets the list of information contained in the declaration of establishment of manufacture or packaging of cosmetic products provided for in Article L. 5131-2 of the Public Health Code, in French.
In the French Public Health Code (CSP), Articles L.5131-1 to L.5131-8 and L.5431-1 to L.5431-9 of Law No. 2014-201 of 24 February 2014 relate to the various provisions for adaptation to European Union law in the field of health (Article 3, I and II) and Articles R.5131-1 to R.5131-15 from decree n° 2015-1417 of 4 November 2015 relating to cosmetic products and tattoo productsany outcome of a process or activity (see also ISO 9000, 3.4.2) and articles R.5431-1 to R.5431-3.
The ICCR (International Cooperation on Cosmetics Regulation) is a voluntary international group of cosmetics regulators aiming to provide the best possible protection for consumers worldwide while minimizing barriers to international trade.
The ANSM (French National Agency for the Safety of Medicines and Public Health) website contains a lot of useful information dedicated, among others, to the market surveillance of cosmetic products (Activities tab) with dedicated pages:
- market surveillance of cosmetic products: the ANSM's professions
- the post-opening period (POP)
- contacts
- the regulation of cosmetic products
- the authorities in charge of cosmetic products
In the ANSM Publications tab, a dedicated page gives access to recommendations of good practice (RBP) concerning optimal strategies for the use of cosmetic products. The pointers are short documents, answering a specific question.
The Cosmetics Observatory is a very rich site with a wide range of information.
Perfumes are regulated by the IFRA Code of Practice. Many national regulations refer to these recommendations, particularly with regard to concentrations and the use of perfumes. In order to comply with these regulations, your fragrance manufacturer must provide you with an IFRA certificate.
1.2 Benefits
Benefits of ISO 22716 certification
Some benefits of ISO 22716 certification:
- preparing and facilitating legal inspections (health authorities)
- validate the compliance of your quality and safety management system with Good Manufacturing Practice (GMP) recommendations
- improved confidence of your customers and customers (ability to deliver safe, healthy and environmentally friendly cosmetic products)
- facilitate access to European and international markets (internationally recognized benchmark)
- your team is made aware around a rewarding project
- always produce products with the quality required for the intended use
- improve the efficiency of your company
- heightened competition
- compliance with legal requirements
L'Oréal, one of the world leaders in the cosmetics industry, has implemented ISO 22716 to guarantee the quality of its products and the safety of consumers. The standardization of procedures and the establishment of a quality culture have made it possible to reduce manufacturing defects and improve the reliability of cosmetic products.
L'Oréal has recorded a significant improvement in the quality of its products, demonstrating the company's commitment to consumer safety and the positive impact of ISO 22716 on financial performance.
1.3 Application
Scope of ISO 22716
Compliance with Good Manufacturing Practices (GMP) concerns the entire branch of the cosmetics industry:
- producers (manufacturers) of finished products
- importers/exporters
- distributors (see Annex 01 and also Articles 6 and 26 of the Cosmetic Regulation)
The processes within the scope of ISO 22716 are manufacturing, storage, packaging, testing and transport.
The end user can be the consumer or a professional (hairdresser, beautician).
Since 11 July 2013, all cosmetic products on the European market are (must be) compliant with Good Manufacturing Practices (GMP), according to the ISO 22716 standard.
The ISO 22716 standard does not apply to research and development activities or to the distribution of finished cosmetic products.
A productany outcome of a process or activity (see also ISO 9000, 3.4.2) intended to be ingested, inhaled, injected or implanted into the human body cannot be considered a cosmetic product.
A cosmetic product may not be represented as having curative or preventive properties with respect to human disease. In this case the productany outcome of a process or activity (see also ISO 9000, 3.4.2) is a medicinal productany outcome of a process or activity (see also ISO 9000, 3.4.2) within the meaning of Article L.5111-1 of the Public Health Code.
But some productsany outcome of a process or activity (see also ISO 9000, 3.4.2) (such as some soaps) can be considered as both cosmetics and drugs at the same time (see the FDA Cosmetics & U.S. Law).
The ISO 22716 standard has been approved and accepted by many global regulatory bodies such as the FDA (Food & Drug Administration), the ICCR (International Cooperation on Cosmetics Regulation) and the European Committee for Standardization (ECS).
1.4 Standards, definitions and books
Standards, definitions and books related to cosmetic products

1.4.1 Standards
Some ISO standards on cosmetics:
- ISO 22716:2007, Cosmetics - Good manufacturing practices (GMP) – Guidelines on Good manufacturing practices
- ISO 17516:2014, Cosmetics - Microbiology - Microbiological limits
- ISO 18416:2015, Cosmetics - Microbiology - Detection of Candida albicans
- ISO 21150:2015, Cosmetics - Microbiology - Detection of Escherichia coli
- ISO 22718:2015, Cosmetics - Microbiology - Detection of Staphylococcus aureus
- ISO 16128-1:2016, Guidelines on technical definitions and criteria for natural and organic cosmetic ingredients and products — Part 1: Definitions for ingredients
- ISO 16128-2:2017, Cosmetics — Guidelines on technical definitions and criteria for natural and organic cosmetic ingredients — Part 2: Criteria for ingredients and products
- ISO 16212:2017, Cosmetics - Microbiology - Enumeration of yeasts and moulds
- ISO 18415:2017, Cosmetics - Microbiology - Detection of specified and unspecified microorganisms
- ISO 21149:2017, Cosmetics - Microbiology - Enumeration and detection of mesophilic aerobic bacteria
- ISO 29621:2017, Cosmetics - Microbiology - Guidelines for risk assessment and identification of products with low microbiological risk
- ISO 11930:2019, Cosmetics - Microbiology - Evaluation of the antimicrobial protection of a cosmetic product
All of these standards and referential can be ordered (in electronic or paper format) on the ISO website.
More than 28,000 standards (in English and other languages) are available free of charge on the Public.resource.Org website.
The ISO 22716 certification is necessary to produce cosmetic products because the Cosmetic Regulation 1223/2009 indicates that the ISO 22716 attestation must be added to the Product Information File (PIF) of your cosmetic product. The placing on the market of a cosmetic product must comply with the rules (requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.1.2)) of the Cosmetic Regulation.
The ISO guide “The integrated use of management system standards” of 2018, contains relevant recommendations on the integration of management systems.
Some useful sites related to cosmetics:
- ANSM - National Agency for the Safety of Medicines and Health Products
- Cosmetic ingredient database - European Commission database for information on cosmetic substances and ingredients
- Ministry of Solidarity and Health France
- DGCCRF - Directorate-General for Competition, Consumer Affairs and Fraud Control
- Le flacon - the composition of your cosmetic products under the magnifying glass
- Cosmetic valley - the world's leading resource centre for cosmetic perfumery in terms of know-how, research and training
- FEBEA - federation of beauty companies
- Ecomundo - experts on chemical substances and their regulatory framework
1.4.2 Definitions
The beginning of wisdom is the definition of terms. Socrates
As seen in paragraph 1.1 ISO 22716 is a standard and at the same time they are guidelines. The verb "should" seems to be more flexible than the verb "shall". But to comply with either verb is to achieve the same end (to meet/fulfill a recommendation or requirementexplicit or implicit need or expectation (see also ISO 9000, 3.1.2)).
Some specific quality and cosmetic terms:
Acceptance criteria: everything that is compared to the requirements to assess compliance
ANSM: National Agency for the Safety of Medicines and Health Products
Audit: a systematic and independent examination to determine whether activities and results meet pre-determined arrangements and are capable of achieving objectives
Batch: quantity of a cosmetic product manufactured in a homogeneous operation cycle
Bulk product (semi-finished): any intermediate product of a process or activity
Calibration: the set of operations for establishing the relationship between the values indicated by the instrument and the known values of a reference standard
Cleaning: any operation to separate and remove dirt by means of chemical, mechanical or temperature action
Conformity: fulfillment of a specified requirement
Contamination: presence of undesirable substances in the product
Control (inspection): actions of measuring, testing, and examining a product, service, process or material to determine compliance with requirements
Corrective action: action to eliminate the causes of nonconformity or any other undesirable event and to prevent their recurrence
Cosmetic product: any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance, protecting them, keeping them in good condition or correcting body odours (Article 2, 1a of Cosmetic Regulation)
CPNP: Cosmetic Products Notification Portal
CSSC: Scientific Committee on Consumer Safety
Customer: anyone who receives a product
Document: any support allowing the treatment of information
Effectiveness: capacity to realize planned activities with minimum effort
Efficiency: financial relationship between achieved results and used resources
Finished product: any end result of a process or activity
Maintenance (preventive): a set of planned preventive actions to maintain e-quipment in perfect condition and ensure the specified service
Making available on the market: any supply of a cosmetic product for distribution, consumption or use on the Community market in the course of a commercial activity, whether in return for payment or free of charge (Article 2, 1g of Cosmetic Regulation)
Management system: set of processes allowing objectives to be achieved
Nonconformity: non-fulfillment of a specified requirement
Organization (company): a structure that satisfies a need
Performance: measurable and expected results of the management system
PIF: product information file
Process: activities that transform inputs into outputs
Product (or service): every result of a process or activity
Quality management: activities allowing the control of a company with regard to quality
Quality: aptitude to fulfill requirements
Recall: any measure aimed at achieving the return of a cosmetic product that has already been made available to the end user (Article 2, 1r of Cosmetic Regulation)
Requirement: explicit or implicit need or expectation
Risk: likelihood of occurrence of a threat or an opportunity
Sanitization: any operation to reduce undesirable invisible contaminants
Subcontractor (supplier): an entity that provides a product
SUE (serious undesirable effect): an undesirable effect which results in temporary or permanent functional incapacity, disability, hospitalization, congenital anomalies or an immediate vital risk or death (article 2, 1p of the Cosmetic Regulation)
Top management: group or persons in charge of the company’s control at the highest level
Undesirable effect: an adverse reaction for human health attributable to the normal or reasonably foreseeable use of a cosmetic product (article 2, 1o of the Cosmetic Regulation)
Waste: anything that is destined for disposal
Withdrawal: any measure aimed at preventing the making available on the market of a cosmetic product in the supply chain (Article 2, 1q of Cosmetic Regulation)
In the terminology of quality management systemsset of processes allowing the achievement of the quality objectives (see also ISO 9000, 3.2.3), do not confuse:
- anomaly, defect, dysfunction, failure, nonconformity, reject and waste:
- anomaly is a deviation from what is expected
- defect is the non-fulfillment of a requirement related to an intended use
- dysfunction is a degraded function that can lead to a failure
- failure is when a function has become unfit
- nonconformity is the non-fulfillment of a requirement in production
- reject is a nonconforming product that will be destroyed
- waste is when there are added costs but no value
- audit program and plan
- an audit program is the annual planning of the audits
- an audit plan is the description of the audit activities
- audit, inspection, auditee and auditor
- an audit is the process of obtaining audit evidence
- an inspection is the conformity verification of a process or product
- an auditee is the one who is audited
- an auditor is the one who conducts the audit
- control and optimize
- control is meeting the objectives
- optimize is searching for the best possible results
- customer, external provider and subcontractor
- a customer receives a product
- an external provider provides a product on which specific work is done
- a subcontractor provides a service or product on which specific work is done
- effectiveness and efficiency
- effectiveness is the level of achievement of planned results
- efficiency is the ratio between results and resources
- follow-up and review
- follow-up is the verification of the obtained results of an action
- review is the analysis of the effectiveness in achieving objectives
- inform and communicate
- to inform is to give someone meaningful data
- to communicate is to pass on a message, to listen to the reaction and discuss
- objective and indicator
- an objective is a sought after commitment
- an indicator is the information on the difference between the pre-set objective and the achieved result
- organization and enterprise, society, company
- organization is the term used by the ISO 9001 standard as the entity between the supplier and the customer
- an enterprise, society and company are examples of organizations
- process, procedure, product, activity and task
- a process is how we satisfy the customer using people to achieve the objectives
- a procedure is the description of how we should conform to the rules
- a product is the result of a process
- an activity is a set of tasks
- a task is a sequence of simple operations
- recall and withdrawal
- recall is a measure to prevent consumption after distribution
- withdrawal is a measure to prevent distribution
Remark 1: the use of ISO 9000, ISO 22716 and Cosmetic Regulation definitions is recommended. The most important thing is to determine a common and unequivocal vocabulary for everyone in the company.
Remark 2: the customer can also be the user, the beneficiary, the trigger, the ordering party or the consumer.
Remark 3: documented information is any information that we must maintain (procedure  ) or retain (record
) or retain (record  ).
).
For other definitions, comments, explanations and interpretations that you don’t find in this module and in annex 06, you can consult: 
- M. Varinia Michalun, Joseph C. DiNardo, Skin Care and Cosmetic Ingredients Dictionary, Milady, 2014
.jpg) Minute of relaxation. Game: Procedure
Minute of relaxation. Game: Procedure
1.4.3 Books
When I think of all the books still left for me to read, I am certain of further happiness. Jules Renard
To go further, here are some books related to cosmetics:
Cosmetics, good manufacturing practices, guidelines on GMPs: the ISO 22716:2007 standard, Questions and answers, 2008 (pdf in French and English)
 Marie Gale, Good Manufacturing Practices for Soap and Cosmetic Handcrafters, Cinnabar Press, 2013
Marie Gale, Good Manufacturing Practices for Soap and Cosmetic Handcrafters, Cinnabar Press, 2013
 André O. Barel et al, Handbook of Cosmetic Science and Technology, CRC Press, 2014
André O. Barel et al, Handbook of Cosmetic Science and Technology, CRC Press, 2014
 Marie Gale, Soap and Cosmetic Labeling: How to Follow the Rules and Regs Explained in Plain English, Cinnabar Press, 2015
Marie Gale, Soap and Cosmetic Labeling: How to Follow the Rules and Regs Explained in Plain English, Cinnabar Press, 2015
 Amparo Salvador et Alberto Chisvert, Analysis of Cosmetic Products, Elsevier Science, 2017
Amparo Salvador et Alberto Chisvert, Analysis of Cosmetic Products, Elsevier Science, 2017
 Suzanne Carpenter, Soap and Cosmetic Packaging & Labeling Rules and Regulations Handbook: How to Implement Good Manufacturing Practices, CreateSpace Independent Publishing Platform, 2017
Suzanne Carpenter, Soap and Cosmetic Packaging & Labeling Rules and Regulations Handbook: How to Implement Good Manufacturing Practices, CreateSpace Independent Publishing Platform, 2017
 Brendan Cooper, GMP Audit Trainer: Good Manufacturing Practices Made Easy, CreateSpace Independent Publishing Platform, 2017
Brendan Cooper, GMP Audit Trainer: Good Manufacturing Practices Made Easy, CreateSpace Independent Publishing Platform, 2017
 Gail Francombe and al, A-Z of Natural Cosmetic Formulation: The definitive beginners’ guide to the essential terminology, theories and ingredient types needed to formulate professional cosmetic products, Goodness & Wonder, 2019
Gail Francombe and al, A-Z of Natural Cosmetic Formulation: The definitive beginners’ guide to the essential terminology, theories and ingredient types needed to formulate professional cosmetic products, Goodness & Wonder, 2019
 John Fauquembergue, SETS UP HIS BIO-COSMETIC BIO-PHARMACEUTICAL COMPANY: Beauty seduces the flesh to get permission to pass to the soul. . ., John, 2020
John Fauquembergue, SETS UP HIS BIO-COSMETIC BIO-PHARMACEUTICAL COMPANY: Beauty seduces the flesh to get permission to pass to the soul. . ., John, 2020
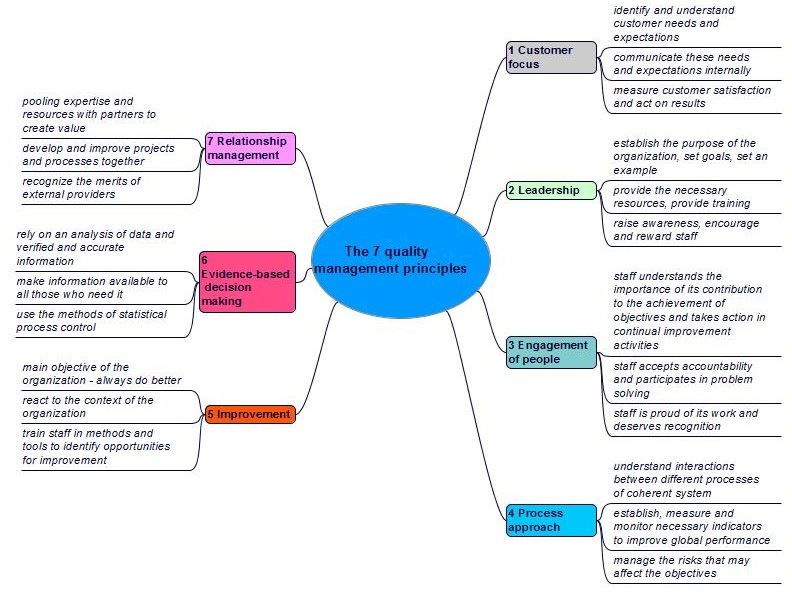
1.5 Principles and steps
Principles of quality management, Deming cycle, requirements ISO 22716, implementation of cosmetic GMP

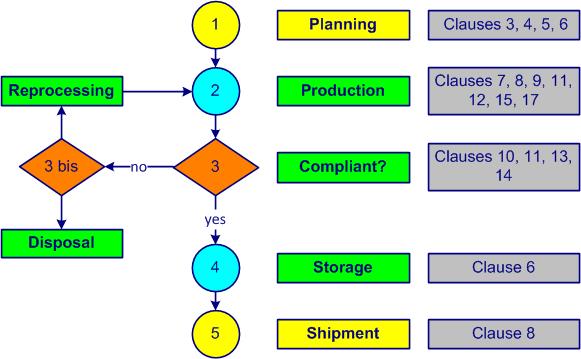
The Deming cycle (figure 1-2) is applied to control any processactivities which transform inputs into outputs (see also ISO 9000, 3.4.1). The PDCA cycles (Plan, Do, Check, Act) are a universal base for continual improvementpermanent process allowing the improvement of the global performance of the organization (see also ISO 9000, 3.2.13 and ISO 14 001, 3.2).
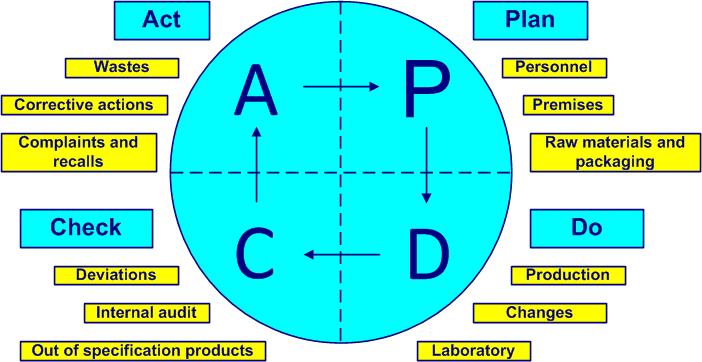
- Plan - planning, managing personnel, premises, raw materials, packaging materials and equipment (ISO 22716, clauses 3, 4, 5 and 6)
- Do - process, manufacture the product, manage finished products, control laboratory activities, manage subcontracting, changes and documentation (ISO 22716, clauses 7, 8, 9, 12, 15 and 17)
- Check - control out-of-specification products, manage deviations and audit (ISO 22716, clauses 10, 13 and 16)
- Act – improve, implement corrective actions, manage waste, complaints and recalls, find new improvements (new PDCA) (ISO 22716 articles 11 and 14)
For more information on the Deming cycle and its 14 points of management theory, you can consult the classic book "Out of the crisis", W. Edwards Deming, MIT press, 1982.
The 311 requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.1.2) (recommendations) of ISO 22716 in clauses 3 to 17 are shown in figure 1-3:
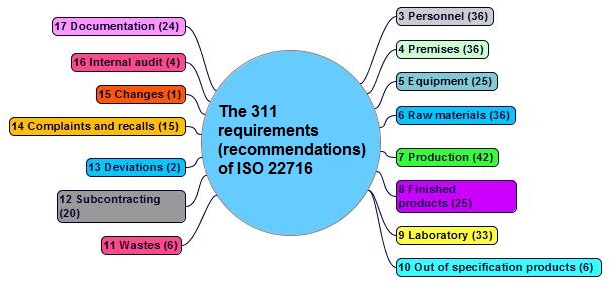
- staff is adequately trained in good manufacturing practices for the production, control and storage of cosmetic products
- the premises ensure the protection of the product and allow efficient maintenance
- the equipment is adapted to the intended use and allows efficient maintenance
- the raw materials and packaging materials meet the acceptance criteria
- the (quality) management system documentation is in place
- raw materials
- packaging materials
- bulk products
- consumables
- finished products
