AS9100D 2016 requirements, aerospace quality management systems
21/08/2017
Quiz requirements AS9100D version 2016 You want to familiarize yourself with the structure of the standard, identify and understand the requirements of AS9100D version 2016, then it's up to you to play!
The "AS9100D version 2016 Requirements" quiz will help you understand the main requirements of the standard.
The questions (requirements) included in this quiz are 113 of the 460 in the standard, but don't worry. These 113 requirements are among the most important, so do not hesitate to learn in a fun way!
Do not think you can finish this quiz in less than an hour, or even two hours, unless of course you are a little genius!
News about AS9100D version 2016
Based on the ISO 9001: 2015 the 460 requirements (verb shall) of clauses 4 to 10 are as follows:
|
No
|
Clause
|
PDCA cycle
|
Requirement No
|
Quantity
|
|
4
|
Context of the organization | Plan | 1 ÷ 30 | 30 |
|
5
|
Leadership | Plan, Do, Check, Act |
31 ÷ 60
|
30
|
|
6
|
Planning | Plan |
61 ÷ 87
|
27
|
|
7
|
Support | Do |
88 ÷ 140
|
53
|
|
8
|
Operation | Do |
141 ÷ 391
|
251
|
|
9
|
Performance evaluation | Check |
392 ÷ 438
|
47
|
|
10
|
Improvement | Act |
439 ÷ 460
|
22
|
|
Total
|
460
|
|||

AS9100D 2016 requirements
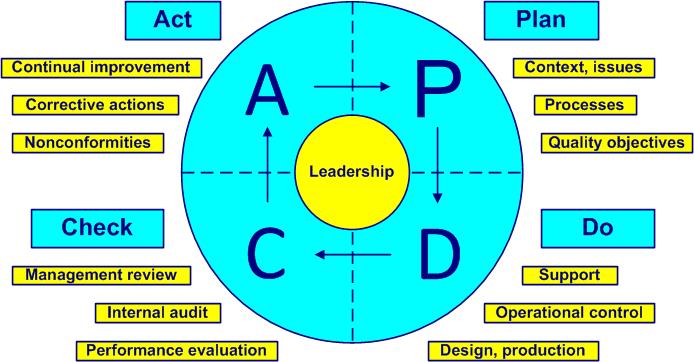
The Deming PDCA cycle
Note 1. Any requirement normally begins with "The organization shall...". For simplicity's sake we present the requirements directly, starting with the verb.
Note 2. The ISO 9001 v 2015 requirements are on a light blue background.
Note 3. Specific AS9100D requirements are on beige background and in italics.
|
No
|
Clause, sub-clause
|
Requirement
|
Comment, link
|
|
| 4 |
Context of the organization
|
|||
| 4.1 |
The organization and its context
|
|||
|
4.1
|
Determine external and internal issues | Understand everything that can influence the purpose and strategic direction of the company (corporate culture, innovation, strategic direction, competition, market, compliance obligation) | ||
|
2 |
4.1 | Monitor and review information about issues | Issue: what one can gain or lose during an activity (factors, conditions) | |
| 4.2 |
Needs and expectations of stakeholders
|
|
||
| 4.2 a | Identify stakeholders | "There is only one valid definition of a business purpose: to create a customer". Peter Drucker. List of relevant stakeholders | ||
| 4 |
4.2 b
|
Clarify the requirements of the stakeholders | Each need and expectation is unique. Aim for a partnership in the long term | |
|
5
|
4.2
|
Monitor and review information about stakeholders and their requirements | Before accepting an order | |
| 4.3 |
Scope of the quality management system
|
|
||
|
6
|
4.3
|
Define the scope of the QMS | Geographic and organizational scope available to stakeholders | |
|
7
|
4.3 a
|
Take into account the external and internal issues | Cf. sub-clause 4.1 | |
|
8
|
4.3 b
|
Take into account the requirements of the stakeholders | Cf. sub-clause 4.2 | |
|
9
|
4.3 c
|
Take into account the products and services | All products and services proposed by the company without exception | |
| 10 |
4.3
|
Apply any requirement of the ISO 9001 standard applicable within the scope of the QMS | The requirements of the standard become internal requirements | |
| 11 | 4.3 | Maintain the scope of the QMS as documented information | Cf. sub-clause 7.5. Include all products and services | |
|
4.3
|
Include in the scope of the QMS justification for any requirements which cannot be met | Every requirement of the ISO 9001 standard which cannot be applied in the company implies a justification | ||
| 4.4 |
Quality management system and its processes
|
|||
|
13
|
4.4.1
|
Establish, implement, maintain and improve a process-based QMS | The company is free to decide how to apply the QMS without forgetting the issues (see sub-clause 4.1) and requirements (see sub-clause 4.2) | |
| 14 | 4.4.1 | Determine the needed processes and their application | "If you cannot describe what you are doing as a process, you do not know what you're doing". Edwards Deming. Process map | |
| 15 |
4.4.1
|
Address also customer and applicable statutory and regulatory requirements |
Cf. sub-clause 8.2.2 | |
|
16
|
4.4.1 a
|
Determine process inputs and outputs | Process sheet | |
| 17 | 4.4.1 b | Determine the sequence and interaction of processes | Flowchart | |
| 18 | 4.4.1 c | Determine the criteria and methods to control processes | Tools of the quality manager | |
| 19 | 4.4.1 d | Determine and ensure the resources | Needed to support processes. Cf. sub-clause 7.1 | |
| 20 | 4.4.1 e | Assign process responsibilities and authorities | Job description of process owners | |
| 21 | 4.4.1 f | Take into account the risks and opportunities for each process | Plan and implement actions to address these risks, cf. sub-clause 6.1 | |
| 22 | 4.4.1 g | Evaluate processes and if necessary modify them | Identify methods to monitor, measure, check and modify processes. Cf. sub-clause 9.1.1 | |
| 23 | 4.4.1 h | Determine the improvement opportunities of processes and the QMS | Cf. sub-clause 10.1 | |
|
24
|
4.4.2 a
|
Maintain documented information on process operation | Cf. sub-clause 7.5. The bare essential is sufficient. Use process map | |
|
25
|
4.4.2 b
|
Retain documented information on process operation | Cf. sub-clause 7.5. The goal is to ensure that processes' results are those planned | |
| 26 |
4.4.2
|
Establish and maintain documented information that includes a general description of relevant stakeholders |
Cf. sub-clause 4.2. a | |
| 27 |
4.4.2
|
Establish and maintain documented information that includes the scope of the QMS | Including boundaries and applicability, cf. sub-clause 4.3 | |
| 28 |
4.4.2
|
Establish and maintain documented information that includes a description of the processes needed for the QMS | And their application in the organization | |
| 29 |
4.4.2
|
Establish and maintain documented information that includes the sequence and interaction of these processes | As sub-clause 4.4.1.b | |
| 30 |
4.4.2
|
Establish and maintain documented information that includes responsibilities and authorities for these processes | As sub-clause 4.4.1.e | |
| 5 |
Leadership
|
|||
| 5.1 |
Leadership and commitment
|
|||
|
|
5.1.1 |
General
|
||
|
31
|
5.1.1 a
|
Assume responsibility for the effectiveness of the QMS | "When you sweep the stairs, you start at the top". Romanian proverb. Top management demonstrates leadership (assumes its responsibility and commitment). Focus on quality and customers | |
| 32 | 5.1.1 b | Establish a quality policy and quality objectives | The policy and the objectives are compatible with strategic direction and context of the company | |
|
33
|
5.1.1 c
|
Integrate QMS requirements in the internal process requirements | Cf. sub-clause 4.4 and sub-clause 7.1.4 | |
|
34
|
5.1.1 d
|
Raise awareness of the process approach and risk-based approach | Cf. sub-clause 0.3 (introduction) and sub-clause 6.1 | |
|
35
|
5.1.1 e
|
Provide the necessary resources for the QMS | Cf. sub-clause 7.1 | |
|
36
|
5.1.1 f
|
Raise awareness on the importance of an effective and conforming QMS | Third quality management principle (engagement of people) | |
|
37
|
5.1.1 g
|
Ensure the achievement of intended results of the QMS | Essential commitment of top management | |
|
38
|
5.1.1 h
|
Support the staff contribution to the effectiveness of the QMS | "Employees first, customers second". Vineet Nayar | |
|
39
|
5.1.1 i
|
Promote continual improvement | Cf. sub-clause 10.3 | |
|
40
|
5.1.1 j
|
Support the leadership of managers | Responsibility and authority of managers are backed at all times by top management | |
|
|
5.1.2
|
Customer focus
|
|
|
|
41
|
5.1.2 a
|
Determine and meet customer, statutory and regulatory requirements | Top management demonstrates leadership (assumes its responsibility and commitment) permanently | |
|
42
|
5.1.2 b
|
Determine and address the potential risks and opportunities | Any risk and opportunity that may influence the conformity of products and services and customer satisfaction. Preserving the goal to always provide compliant products and services | |
|
43
|
5.1.2 c
|
Maintain the objective of better satisfying the customer | First quality management principle (customer focus) | |
| 44 |
5.1.2 d
|
Ensure product and service conformity and on-time delivery | After measurement, appropriate action is taken if planned results are not, or will not be, achieved | |
| 5.2 |
Policy
|
|||
| 5.2.1 |
Establishing the quality policy
|
|
||
|
45
|
5.2.1 a
|
Establish, implement and maintain a suitable quality policy | Top management applies a policy adapted to the purpose, strategic direction, culture and business context | |
| 46 | 5.2.1 b | Provide a framework to define and review the quality objectives | Cf. sub-clause 6.2 | |
| 47 | 5.2.1 c | Include meeting the applicable requirements | Cf. sub-clause 9.1.3 | |
| 48 | 5.2.1 d | Include a commitment to continuously improve the QMS | Cf. sub-clause 10.3 | |
| 5.2.2 |
Communicating the quality policy
|
|
||
| 49 | 5.2.2 a | Maintain the quality policy as documented information | And make it available inside the company. Cf. sub-clause 7.5 | |
| 50 | 5.2.2 b | Communicate the quality policy | So it is understand and applied, cf. sub-clause 7.4 | |
| 51 | 5.2.2 c | Keep the quality policy available | The quality policy cannot be a confidential document, it is available to relevant stakeholders | |
|
5.3
|
Roles, responsibilities, authorities
|
|
||
|
52
|
5.3
|
Define and communicate the responsibilities and authorities | Top management assigns all relevant roles in the QMS | |
|
53
|
5.3 a
|
Define and communicate the responsibilities and authorities | Top management assigns all relevant roles according to the requirements of the ISO 9001 standard | |
|
54
|
5.3 b
|
Define and communicate the responsibilities and authorities | Top management assigns all relevant roles so that processes deliver expected results | |
|
55
|
5.3 c
|
Define and communicate the responsibilities and authorities | Top management assigns all relevant roles so that reporting on the performance of the QMS and improvement opportunities is done, cf. sub-clause 10.1 | |
|
56
|
5.3 d
|
Define and communicate the responsibilities and authorities | Top management assigns all relevant roles so that customer focus is ensured (first quality management principle) | |
|
57
|
5.3 e
|
Define and communicate the responsibilities and authorities | Top management assigns all relevant roles so that implemented changes to the QMS do not affect its integrity | |
| 58 |
5.3
|
Appoint a management representative |
He is memeber of the organization's management | |
| 59 |
5.3
|
Have the responsibility and authority to establish and maintain the QMS |
Cf. sub-clause 4.4.1 | |
| 60 |
5.3
|
Have the organizational freedom and unrestricted access to top management |
To resolve all problems related with quality management | |
|
6
|
Planning
|
|||
| 6.1 |
Actions to address risks and opportunities
|
|||
| 61 |
6.1.1 a
|
Take into account risks and opportunities | In order to ensure that the QMS can achieve its expected results, cf. sub-clauses 4.1 (context) and 4.2 (stakeholders). "Any decision involves a risk". Peter Barge | |
| 62 | 6.1.1 b | Take into account opportunities | In order to increase the desirable effects (positive impact) | |
|
63
|
6.1.1 c
|
Take into account risks | In order to reduce the undesirable effects (negative impacts) | |
| 64 | 6.1.1 d | Take into account risks and opportunities | In order to confirm the approach of continual improvement, cf. sub-clause 10.3 | |
| 65 | 6.1.2 a | Plan actions to address risks and opportunities | Take into account risks in every process | |
| 66 | 6.1.2 b 1 | Plan the way to implement actions | Define how to integrate actions in the QMS processes, cf. sub-clause 4.4 | |
| 67 | 6.1.2 b 2 | Plan the way to evaluate actions | Follow the results of each action | |
| 68 | 6.1.2 | Adapt actions to risks and opportunities | Compared to the potential impact on the conformity of products and services | |
|
6.2
|
Quality objectives
|
|
||
|
69
|
6.2.1
|
Establish quality objectives for processes | "He who has no goals will not achieve them". Sun Tzu | |
| 70 | 6.2.1 a | Choose quality objectives | Clarify criteria for setting objectives that are consistent with the quality policy | |
|
71
|
6.2.1 b
|
Use measurable objectives | And realistic | |
|
72
|
6.2.1 c
|
Consider applicable requirements | Cf. sub-clause 4.2 | |
| 73 | 6.2.1 d | Adopt relevant objectives | In order to ensure the conformity of products and services and improved customer satisfaction | |
| 74 | 6.2.1 e | Monitor objectives | Frequently | |
|
75
|
6.2.1 f
|
Communicate on objectives | At all levels | |
| 76 | 6.2.1 g | Update objectives | During management review, cf. sub-clause 9.3 | |
| 77 |
6.2.1
|
Maintain documented information on the quality objectives | Cf. sub-clause 7.5 | |
| 78 |
6.2.2 a
|
Plan how to do | In order to achieve quality objectives | |
| 79 |
6.2.2 b
|
Plan necessary resources | In order to achieve quality objectives | |
| 80 |
6.2.2 c
|
Plan responsibilities | In order to achieve quality objectives | |
| 81 |
6.2.2 d
|
Plan deadlines | In order to achieve quality objectives | |
| 82 |
6.2.2 e
|
Plan the way to evaluate results | In order to achieve quality objectives | |
| 6.3 |
Planning of changes
|
|||
|
83
|
6.3 | Plan the need for changes of the QMS | "The only person who likes change is a wet baby". Cf. sub-clause 4.4 | |
|
84
|
6.3 a | Plan the changes | Taking into account the purpose of the change and the possible consequences | |
|
85
|
6.3 b | Plan the changes | Taking into account the maintenance of the integrity of the QMS | |
|
86
|
6.3 c | Plan the changes | Taking into account the available resources | |
|
87
|
6.3 d | Plan the changes | Taking into account the assigned responsibilities and authorities | |
| 7 |
Support
|
|||
| 7.1 |
Resources
|
|
||
|
|
7.1.1
|
General
|
|
|
|
88
|
7.1.1 | Provide the necessary resources | In order to support the QMS | |
|
89
|
7.1.1 a | Take into account existing resources | And their capabilities and constrains | |
|
90
|
7.1.1 b | Take into account the need for the use of external providers | In order to provide necessary services not available inside the company | |
|
|
7.1.2 |
People
|
|
|
|
91
|
7.1.2 | Provide suitable people for the effective operation of the QMS and its processes | "But in the long run - and I emphasize this - no matter how good or successful you are or how clever or crafty, your business and its future are in the hands of the people you hire". Akio Morita | |
|
7.1.3
|
Infrastructure | |||
|
92
|
7.1.3 | Provide and maintain the infrastructure necessary to the functioning of processes | In order to achieve conformity of products and services. Examples: buildings, equipment, transportation, hardware, software | |
|
7.1.4
|
Process environment | |||
|
93
|
7.1.4 | Provide and maintain the suitable environment necessary to the functioning of processes | In order to achieve conformity of products and services. Examples: corporate culture, work atmosphere, temperature, ergonomics | |
| 7.1.5 |
Monitoring and measuring resources
|
|
||
| 7.1.5.1 |
General
|
|
||
|
94
|
7.1.5.1 | Provide suitable monitoring and measuring resources | In order to obtain expected inspection results | |
|
95
|
7.1.5.1 a
|
Provide adequate resources to the specific inspections | To inspect is to monitor and measure. Cf. sub-clause 7.2 | |
| 96 | 7.1.5.1 b | Maintain resources | In order to ensure fitness for their purpose | |
| 97 | 7.1.5.1 | Retain documented information on the adequacy of inspection resources | Cf. sub-clause 7.5 | |
| 7.1.5.2 |
Measurement traceability
|
|
||
| 98 | 7.1.5.2 a | Verify or calibrate regularly the measuring equipment | In order to have confidence in the measurement results. When no such standards exist retain documented information on the reference used, cf. sub-clause 7.5 | |
| 99 | 7.1.5.2 b | Identify the measuring equipment | In order to monitor the validity of their calibration (or verification) | |
| 100 | 7.1.5.2 c | Protect the measuring equipment | Against activities that may invalidate the results of the measurement (settings or deterioration) | |
| 101 | 7.1.5.2 | Conduct corrective action on previous measurement results | When the verification or calibration of a measuring instrument is not in conformity | |
| 102 |
7.1.5.2
|
Establish, implement and maintain a process for the recall of monitoring and measuring equipment |
When calibration or verification are required | |
| 103 |
7.1.5.2
|
Maintain a register of the monitoring and measuring equipment |
List of equipment used including software, tester, personally owned and customer supplied equipment | |
| 104 |
7.1.5.2
|
Include in the register specific equipment data |
Data such as type, unique identification, location, calibration or verification method, frequency and acceptance criteria | |
| 105 |
7.1.5.2
|
Ensure suitable environmental conditions for carrying out calibration or verification |
Cf. sub-clause 7.1.4 | |
|
|
7.1.6
|
Organizational knowledge | ||
| 106 | 7.1.6 | Determine the necessary knowledge | In order to control the processes and the conformity of products and services | |
| 107 | 7.1.6 | Acquire, maintain and make organizational knowledge available to the extend necessary | In order to maintain the performance of the QMS | |
| 108 | 7.1.6 | Take into account the need for additional knowledge | When needs and trends have changed | |
| 7.2 |
Competence
|
|||
| 109 | 7.2 a | Determine the necessary competence | Clarify quality competence requirements. Identify people who have an influence on the quality performance | |
| 110 | 7.2 b | Ensure the competence | Which are based on appropriate training or experience | |
| 111 | 7.2 c | Undertake activities to acquire the necessary competence and evaluate the effectiveness of these activities | Training, coaching, external providers | |
| 112 | 7.2 d | Retain documented information on staff competence | Cf. sub-clause 7.5 | |
| 7.3 | Awareness |
|
||
| 113 | 7.3 a | Ensure the staff is aware of the quality policy | Including people who carry out work under the company's control. Cf. sub-clause 5.2 | |
| 114 | 7.3 b | Ensure the staff is aware of the quality objectives | Cf. sub-clause 6.2 | |
| 115 | 7.3 c | Ensure the staff is aware of its contribution | In order to improve the performance of the QMS | |
| 116 | 7.3 d | Ensure the staff is aware of negative impacts | If QMS requirements are not met | |
| 117 |
7.3 e
|
Make staff aware of QMS documented information |
Including changes implemented, cf. sub-clause 7.5 | |
| 118 |
7.3 f
|
Make staff aware of their contribution to product or service conformity |
Cf. sub-clause 9.1.2 | |
| 119 |
7.3 g
|
Make staff aware of their contribution to product safety | Cf. sub-clause 8.1.3 | |
| 120 |
7.3 h
|
Make staff aware of the importance of ethical behavior | Corporate culture, happiness at work | |
| 7.4 |
Communication
|
|||
| 121 | 7.4 a | Define the subjects on which to communicate | Internally and externally. "Good news walks, bad news runs". Swedish proverb | |
| 122 | 7.4 b | Define when to communicate | Respond quickly to claims | |
| 123 | 7.4 c | Define with whom to communicate | Communication goes both ways | |
|
124
|
7.4 d
|
Define how to communicate | Orally, in writing; Internet, video | |
|
125
|
7.4 e
|
Assign who will communicate | The one who is closest to the subject | |
| 7.5 |
Documented information
|
|||
| 7.5.1 |
General
|
|||
| 126 | 7.5.1 a | Include the documented information required by the ISO 9001 standard | Documented information to maintain (documented procedures) :
|
|
| 127 | 7.5.1 b | Select the documented information deemed necessary to the effectiveness of the QMS | "Spoken words fly away, written one stay". Latin proverb | |
| 7.5.2 |
Creating and updating
|
|||
| 128 | 7.5.2 a | Create, identify and describe the documented information | Codification, title, author, subject, product | |
| 129 | 7.5.2 b | Choose the format and the media of the documented information | Language, graphics; paper, electronic | |
| 130 | 7.5.2 c | Review and approve the adequacy of the documented information | Who writes, codifies, who approves | |
| 7.5.3 |
Control of documented information
|
|||
| 131 | 7.5.3.1 a | Control the availability of the documented information | Where and when required in a form that is suitable for use | |
| 132 | 7.5.3.1 b | Control the protection of the documented information | Loss of confidentiality, loss of integrity, misuse | |
| 133 | 7.5.3.2 a | Control the distribution, access and use of the documented information | Who is in charge, method to use, rule to follow | |
| 134 | 7.5.3.2 b | Control the storage of the documented information | Including preservation, protection and readability | |
| 135 | 7.5.3.2 c | Control the changes of the documented information | Use of updated versions, restricted access to obsolete versions | |
| 136 | 7.5.3.2 d | Control the retention time and the removal of the documented information | Retention period, disposal method | |
| 137 |
7.5.3.2 e
|
Prevent unintended use of obsolete documented information |
By removal, by placing them out of reach or by applying an appropriate identification | |
| 138 | 7.5.3.2 | Control the documented information of external origin | Unique codification, access, protection | |
| 139 | 7.5.3.2 | Protect the documented information | Who has the right to read, who has the right to change | |
| 140 |
7.5.3.2
|
Define a process for the protection of documented information in electronic form |
Automatic backup (every night), change rights established and respected | |
| 8 |
Operation
|
Do | ||
| 8.1 |
Operational planning and control
|
|||
| 141 | 8.1 a | Plan and determine the requirements for the products and services | By controlling processes. Cf. sub-clauses 4.4 et 6.1 | |
| 142 | 8.1 b 1 | Establish the criteria | For processes | |
| 143 | 8.1 b 2 | Establish the criteria | For the acceptance of conforming products and services | |
| 144 | 8.1 c | Determine necessary resources | Needed to achieve conformity of products and services | |
| 145 | 8.1 d | Control the processes | In accordance with the criteria of sub-clauses 8.1 b 1 and 8.1 b 2 | |
| 146 | 8.1 e 1 | Determine, maintain and retain the documented information on process control | To have confidence that the process results are as expected. Cf. sub-clause 7.5 | |
| 147 | 8.1 e 2 | Determine, maintain and retain the documented information on product and service conformity | Meet applicable requirements. Cf. sub-clause 7.5 | |
| 148 |
8.1 f
|
Determine the processes and controls needed to manage critical items |
Including production process inspections when key characteristics are modified | |
| 149 |
8.1 g
|
Engage representatives of affected functions |
In order to ensure operational planning and control | |
| 150 |
8.1 h
|
Determine the processes and resources |
In order to support the use and maintenance of the products and services | |
| 151 |
8.1 i
|
Determine the products and services to be obtained from external providers |
Cf. sub-clause 8.4 | |
| 152 |
8.1 j
|
Establish the controls needed |
To prevent the delivery of nonconforming products and services | |
| 153 |
8.1
|
Plan and manage product and service provision in a structured and controlled manner |
Including scheduled events with an acceptable risk within resource and schedule constraints. In other words, project planning and management or program management | |
| 154 | 8.1 | Adapt planning outputs to internal operating modes | Cf. sub-clauses 4.4 and 6.1 | |
|
155 |
8.1 | Control planned and unplanned changes | Analyze the consequences of unplanned changes, actions to limit the effects | |
|
156 |
8.1 | Control the outsourced processes | Cf. sub-clause 8.4 | |
| 157 |
8.1
|
Establish, implement and maintain a process to transfer of work |
In order to ensure continuing conformity of the work to requirements | |
| 158 |
8.1
|
Manage work transfer impacts and risks |
Cf. sub-clause 8.1.1 | |
| 8.1.1 |
Operational risk management
|
|||
| 159 |
8.1.1 a
|
Plan, implement and control a process for managing operational risks by assignment of responsibilities |
As appropriate to the company and the products and services. The risk being considered as the probability of occurrence and the severity of the consequences | |
| 160 |
8.1.1 b
|
Plan, implement and control a process for managing operational risks by definition of risk assessment criteria | Such as likelihood, consequences, risk acceptance | |
| 161 |
8.1.1 c
|
Plan, implement and control a process for managing operational risks by identification, assessment and communication of risks | Throughout product realization | |
| 162 |
8.1.1 d
|
Plan, implement and control a process for managing operational risks by identification, implementation and management of actions | In order to mitigate risks that exceed acceptance criteria | |
| 163 |
8.1.1 e
|
Plan, implement and control a process for managing operational risks by acceptance of residual risks | After implementation of mitigating actions | |
| 8.1.2 |
Configuration management
|
|
||
| 164 |
8.1.2
|
Plan, implement and control a process for configuration management |
As appropriate to the company and its products and services in order to ensure the identification and control of physical and functional attributes throughout the product lifecycle | |
| 165 |
8.1.2 a
|
Control product identity and traceability |
Including the implementation of identified changes | |
| 166 |
8.1.2 b
|
Ensure that the documented information is consistent with the actual attributes of the products and services |
Documented information on requirements, design, verification, validation and acceptance | |
| 8.1.3 |
Product safety
|
|||
| 167 |
8.1.3
|
Plan, implement and control the processes to ensure product safety during the product life cycle as appropriate to the company and the product | Such as hazard identification, management of associated risks, management of safety critical items, analysis and reporting of occurred safety-related events, safety training of staff | |
| 8.1.4 |
Prevention of counterfeit parts
|
|||
| 168 |
8.1.4
|
Plan, implement and control processes for the prevention of counterfeit parts |
Including suspicion of use or their inclusion in product delivered to the customer. Consider staff training and awareness, application of a parts obsolescence monitoring program, controls for acquiring externally provided product from original or authorized manufacturers and distributors, traceability requirements for parts and components, verification and tests to detect counterfeit parts, monitoring of reports from external sources, quarantine of suspected or detected parts | |
| 8.2 |
Requirements for products and services
|
|||
| 8.2.1 |
Customer communication
|
|||
| 169 | 8.2.1 a | Provide information to customers | Related to products and services | |
| 170 | 8.2.1 b | Control communication with customers | Related to contracts, orders, changes and consultations | |
| 171 | 8.2.1 c | Control communication with customers | Regarding the perception, opinions, complaints and recommendations. "The most important thing in communication is hearing what is not said". Peter Drucker | |
| 172 | 8.2.1 d | Control communication with customers | Regarding their property. Cf. sub-clause 8.5.3 | |
| 173 | 8.2.1 e | Control communication with customers | Regarding specific requirements for contingency actions | |
| 8.2.2 |
Determining the requirements related to products and services
|
|||
| 174 | 8.2.2 a 1 | Develop specific activities for products and services | In order to clarify statutory and regulatory requirements | |
| 175 | 8.2.2 a 2 | Define internal requirements | Related to processes, products and services. And check that the requirements can be met | |
| 176 | 8.2.2 b | Be able to respond to claims | In a relevant way (with facts) | |
| 177 |
8.2.2 c
|
Determine special requirements for products and services |
High level risks. Take into account the complexity of the product and the process, the performance requirements at the limit of technical possibilities | |
| 178 |
8.2.2 d
|
Identify operational risks |
Cf. sub-clause 8.1.1. Such as new technology, short delivery time frame, ability and capacity to provide | |
| 8.2.3 | Review of requirements related to products and services |
|
||
| 179 | 8.2.3.1 | Be able to respond to customers | Regarding requirements of proposed products and services | |
| 180 | 8.2.3.1 a | Review explicit customer requirements | Before order acceptance. Including delivery and post-delivery activities requirements | |
| 181 | 8.2.3.1 b | Review implicit customer requirements | Before order acceptance. Unformulated requirements but necessary for specified use or use intended by the customer | |
| 182 |
8.2.3.1 c
|
Review internal requirements | Between requirements of an order and those previously expressed | |
| 183 |
8.2.3.1 d
|
Review statutory and regulatory requirements | Applicable to the products and services | |
| 184 | 8.2.3.1 e | Review gaps | Between requirements of an order (or contract) and those previously expressed | |
| 185 | 8.2.3.1 | Resolve gaps | Before order acceptance and commitment to provide products and services | |
| 186 |
8.2.3.1
|
Coordinate the review | With functions concerned | |
| 187 |
8.2.3.1
|
Negotiate a mutually acceptable requirement with the customer |
When the review shows that some customer requirements cannot be met or can only partially be met | |
| 188 | 8.2.3.1 | Confirm requirements before accepting an order | When requirements are not documented | |
| 189 | 8.2.3.2 a | Retain the documented information on the results of the reviews of requirements | Cf. sub-clause 7.5 | |
| 190 | 8.2.3.2 b | Retain the documented information on any new or changed requirement for the products and services | Cf. sub-clause 7.5 | |
| 8.2.4 | Changes to requirements for products and services |
|
||
| 191 | 8.2.4 | Communicate changes to relevant persons | After changing requirements in the documented information | |
| 8.3 |
Design and development of products and services
|
|||
| 8.3.1 |
General
|
|||
| 192 | 8.3.1 | Establish, implement and maintain a process of design and development | When the product or service requirements are not yet defined. "I have not failed. I just found 10,000 ways that will not work". Thomas Edison | |
| 8.3.2 | Design and development planning | |||
| 193 | 8.3.2 a | Plan the design and development stages | Taking into account the specificity of design and development activities | |
| 194 | 8.3.2 b | Plan the design and development stages | Taking into account the process requirements and applicable reviews | |
| 195 | 8.3.2 c | Plan the design and development stages | Taking into account the verification and validation activities | |
| 196 | 8.3.2 d | Plan the design and development stages | Taking into account the necessary responsibilities and authorities | |
| 197 | 8.3.2 e | Plan the design and development stages | Taking into account the needs of internal and external resources | |
| 198 | 8.3.2 f | Plan the design and development stages | Taking into account the relations between persons participating in the design and development | |
| 199 | 8.3.2 g | Plan the design and development stages | Taking into account the need for involvement of customers and users | |
| 200 | 8.3.2 h | Plan the design and development stages | Taking into account the requirements for subsequent products and services | |
| 201 | 8.3.2 i | Plan the design and development stages | Taking into account the level of control expected by stakeholders | |
| 202 | 8.3.2 j | Plan the design and development stages | Taking into account the documented information meeting design and development requirements. Cf. sub-clauses 8.3.3, 8.3.5 and 8.3.6 | |
| 203 |
8.3.2
|
Divide, when appropriate, the design and development effort into distinct activities |
For each activity define the tasks, necessary resources, responsibilities , inputs and outputs | |
| 204 |
8.3.2
|
Consider the ability to provide, verify, test and maintain products and services |
Cf. sub-clause 8.1. a | |
| 8.3.3 |
Design and development inputs
|
|||
| 205 | 8.3.3 | Determine essential requirements | On specific types of products and services from design and development | |
| 206 | 8.3.3 a | Determine functional requirements | Taking into account also the performance requirements | |
| 207 | 8.3.3 b | Clarify inputs | Taking into account the information from similar activities | |
| 208 | 8.3.3 c | Clarify inputs | Taking into account the statutory and regulatory requirements | |
| 209 | 8.3.3 d | Clarify inputs | Taking into account the corporate culture, internal rules of art | |
| 210 | 8.3.3 e | Clarify inputs | Taking into account the potential consequences of product and service failure | |
| 211 |
8.3.3 f
|
Take into account the potential consequences of obsolescence |
Such as consequences related to materials, processes, components, equipment, products | |
| 212 | 8.3.3 | Check that the input items are complete and unambiguous | In order to realize suitable design and development process | |
| 213 | 8.3.3 | Resolve potential conflicts between inputs | In order to obtain complete and unambiguous inputs | |
| 214 | 8.3.3 | Retain the documented information on design and development inputs | Cf. sub-clause 7.5 | |
| 8.3.4 |
Design and development controls
|
|||
| 215 | 8.3.4 a | Define clearly the expected results | Regarding processes, products and services | |
| 216 | 8.3.4 b | Conduct reviews as planned | Regarding processes, products and services | |
| 217 | 8.3.4 c | Check that outputs meet input requirements | Cf. sub-clause 8.3.5 | |
| 218 | 8.3.4 d | Validate products and services | To ensure that the specified application requirements or those for the intended use are satisfied | |
| 219 | 8.3.4 e | Take actions in response to identified problems | During reviews, verifications and validations | |
| 220 | 8.3.4 f | Ensure that the documented information is retained | Cf. sub-clause 7.5. Cf. sub-clauses 8.3.3, 8.3.5 and 8.3.6 | |
| 221 |
8.3.4 g
|
Authorize progression to the next stage |
Before beginning next stage | |
| 222 |
8.3.4 h
|
Include representatives of functions concerned |
For the review of design and development stages | |
| 223 |
8.3.4.1 a
|
Plan, control, review and document tests necessary for verification and validation |
In order to ensure and prove that the tests or specifications identify the product to be tested and the resources to be used, define test objectives and conditions, parameters to be recorded and relevant acceptance criteria | |
| 224 |
8.3.4.1 b
|
Ensure and prove that test procedures describe the test methods to be used |
And also how to perform the test and how to record the results | |
| 225 |
8.3.4.1 c
|
Ensure and prove that the configuration of the test item is correct | Cf. sub-clause 8.1.2 | |
| 226 |
8.3.4.1 d
|
Ensure and prove that the requirements of the test plan are observed | And also test procedures | |
| 227 |
8.3.4.1 e
|
Ensure and prove that the acceptance criteria are met | Also for the tests necessary for verification and validation | |
| 228 |
8.3.4.1
|
Control monitoring and measuring devices used for testing |
Cf. sub-clause 7.1.5 | |
| 229 |
8.3.4.1
|
Ensure that reports, calculations, test results, and others are able to demonstrate that the design for the product or service meets the specification requirements for all identified operational conditions | At the completion of design and development | |
| 8.3.5 | Design and development outputs | |||
| 230 | 8.3.5 a | Ensure that outputs meet input requirements | Cf. sub-clause 8.3.3 | |
| 231 | 8.3.5 b | Ensure that outputs are in line with the subsequent processes | Regarding the products and services | |
| 232 | 8.3.5 c | Ensure that outputs include monitoring and measuring requirements | Including acceptance criteria | |
| 233 | 8.3.5 d | Ensure that outputs are suitable for their intended use | Proper use or planned by the customer in complete safety | |
| 234 |
8.3.5 e
|
Specify, as applicable, any critical items and concerned actions to be taken |
Including key characteristics | |
| 235 |
8.3.5 f
|
Ensure that outputs are approved by authorized person |
Prior to release | |
| 236 |
8.3.5
|
Define the data required to allow the product to be identified, manufactured, verified, used and maintained |
Such as drawings, part lists needed to define the configuration and the design characteristics, materials, processes, manufacturing and assembly data, handling, packaging and preservation to ensure conforming product or service, technical data for repair and maintenance | |
| 237 | 8.3.5 | Retain the documented information on outputs | Cf. sub-clause 7.5 | |
|
|
8.3.6 |
Design and development changes
|
||
| 238 | 8.3.6 | Identify, review and control the changes made to inputs and outputs | To ensure that the changes have no impact on meeting the requirements | |
| 239 |
8.3.6
|
Implement a process with criteria for notifying its customer about changes that affect customer requirements |
Prior to implementation | |
| 240 | 8.3.6 a | Retain the documented information on changes | Cf. sub-clause 7.5 | |
| 241 | 8.3.6 b | Retain the documented information on results of reviews | Cf. sub-clause 7.5 | |
| 242 | 8.3.6 c | Retain the documented information on who authorized the changes | Cf. sub-clause 7.5 | |
| 243 | 8.3.6 d | Retain the documented information on actions | In order to prevent negative impacts. Cf. sub-clause 7.5 | |
| 244 |
8.3.6
|
Control design and development changes in accordance with the configuration management process |
Cf. sub-clause 8.1.2 | |
| 8.4 |
External providers
|
|||
| 8.4.1 |
General
|
|||
| 245 | 8.4.1 | Ensure that the outputs of external providers meet specified requirements | "You can outsource the activity, but you cannot outsource risk". Michael Gallagher | |
| 246 |
8.4.1
|
Be responsible for the conformity of all externally provided processes, products and services |
Including from sources defined by the customer | |
| 247 |
8.4.1
|
Ensure, when required, that customer-designated or approved external providers are used |
Including process sources e.g. special processes | |
| 248 |
8.4.1
|
Identify and manage the risks associated with the external provision of processes, products and services |
Including the selection and use of external providers | |
| 249 |
8.4.1
|
Require that external providers apply appropriate controls to their direct and sub-tier external providers |
To ensure that requirements are met | |
| 250 | 8.4.1 a | Apply the requirements for the control of products and services provided by external providers | When the products and services are integrated internally | |
| 251 | 8.4.1 b | Apply the requirements for the control of products and services | When the products and services are provided directly to customers by external providers on behalf of the company | |
| 252 | 8.4.1 c | Apply the requirements for the control of process done by external providers | When a decision has been made to outsource the process | |
| 253 | 8.4.1 | Establish and implement evaluation and selection criteria of external providers and monitor their performance | Including regular re-evaluation | |
| 254 | 8.4.1 | Retain the documented information on the results of the evaluation and monitoring | Cf. sub-clause 7.5 | |
| 255 |
8.4.1.1 a
|
Define the process, responsibilities and authority for the approval status decision, changes of the approval status |
In order to use external providers depending on their approval status | |
| 256 |
8.4.1.1 b
|
Maintain a register of its external providers that includes approval status (approved, conditional, disapproved) |
Including the scope of the approval such as product type, process family | |
| 257 |
8.4.1.1 c
|
Periodically review external provider performance |
Such as process, product and service conformity, on-time delivery performance | |
| 258 |
8.4.1.1 d
|
Define the necessary actions to take when dealing with external providers that do not meet requirements |
Cf. sub-clauses 8.7 and 10.2 | |
| 259 |
8.4.1.1 e
|
Define the requirements for controlling documented information created or retained by external providers |
Cf. sub-clause 7.5 | |
| 8.4.2 |
Type and extent of control
|
|||
| 260 | 8.4.2 | Ensure the level of control of external providers on meeting the requirements | In order that the provision of external providers do not affect the conformity of products and services delivered to the customer | |
| 261 | 8.4.2 a | Ensure that the processes of external providers are controlled | In conformity with the external service provider QMS. Any outsourced process is included in the scope of the QMS | |
| 262 | 8.4.2 b | Define how to control the external provider and its process outputs | The level of control (or influence) of an external service provider is sometimes a very sensitive area. Stay alert and caring! | |
| 263 | 8.4.2 c 1 | Take into account the potential impact of the outputs of the external provider | On meeting the requirements of products and services delivered to the customer and on statutory and regulatory requirements | |
| 264 | 8.4.2 c 2 | Take into account the control of the external provider | And effectiveness of this control | |
| 265 |
8.4.2 c 3
|
Take into account the results of the periodic review of external provider performance |
Cf. sub-clause 8.4.1.1 c | |
| 266 | 8.4.2 d | Define how to control the outputs of externally provided processes | Verification and other activities necessary to ensure that the provision of external providers does not affect the conformity of products and services delivered to the customer | |
| 267 |
8.4.2
|
Perform verification activities of externally provided processes, products and services according to the risks identified |
Cf. sub-clause 8.1.1. Audit at the external provider's premises, review of required documentation, review of production part approval process, product inspection and service verification upon receipt | |
| 268 |
8.4.2
|
Include inspection or periodic testing, as applicable, when there is high risk of nonconformities |
Including counterfeit parts | |
| 269 |
8.4.2
|
Identify and record in order to allow recall and replacement if later it is found that the product does not meet requirements |
When externally provided product is released for production use, pending completion of all required verification activities | |
| 270 |
8.4.2
|
Define the scope and requirements for delegation for verification activities to the external provider |
Cf. sub-clause 7.5. Maintain a register of delegations | |
| 271 |
8.4.2
|
Periodically monitor the external provider's delegated verification activities |
And record the results | |
| 272 |
8.4.2
|
Implement a process to evaluate the data in the test reports to confirm that the product meets requirements |
When test reports are utilized to verify externally provided products | |
| 273 |
8.4.2
|
Implement a process to validate the accuracy of test reports |
When a customer (or organization) has identified raw material as significant operational risk (critical items) | |
| 8.4.3 |
Information for external providers
|
|||
| 274 | 8.4.3 | Check the adequacy of the requirements | And only after communicate them to the external provider | |
| 275 | 8.4.3 a | Communicate to external providers the requirements | Regarding the processes, products and services to provide | |
| 276 | 8.4.3 b 1 | Communicate to external providers the requirements | Regarding the approval of products and services | |
| 277 | 8.4.3 b 2 | Communicate to external providers the requirements | Regarding the approval of methods, processes and equipment | |
| 278 | 8.4.3 b 3 | Communicate to external providers the requirements | Regarding the approval of the release of products and services | |
| 279 | 8.4.3 c | Communicate to external providers the requirements | Regarding the competence (including required qualifications) | |
| 280 | 8.4.3 d | Communicate to external providers the requirements | Regarding the relations between the external provider and the company | |
| 281 | 8.4.3 e | Communicate to external providers the requirements | Regarding the control and monitoring of the external provider's performance | |
| 282 | 8.4.3 f | Communicate to external providers the requirements | Regarding the verification or validation activities that the company or its customer intends to realize at the external provider's premises | |
| 283 |
8.4.3 g
|
Communicate to external providers the requirements |
Regarding design and development control | |
| 284 |
8.4.3 h
|
Communicate to external providers the requirements | Regarding special requirements, critical items or key characteristics | |
| 285 |
8.4.3 i
|
Communicate to external providers the requirements | Regarding test, inspection, and verification, including production process verification | |
| 286 |
8.4.3 j
|
Communicate to external providers the requirements | Regarding the use of statistical techniques for product acceptance and related instructions for acceptance by the organization | |
| 287 |
8.4.3 k
|
Communicate to external providers the requirements | Regarding the need to implement a QMS | |
| 288 |
8.4.3 k
|
Communicate to external providers the requirements | Regarding the need to use customer-designated or approved external providers, including process sources | |
| 289 |
8.4.3 k
|
Communicate to external providers the requirements | Regarding the need to notify the organization of nonconforming processes, products or services and obtain approval for their disposition | |
| 290 |
8.4.3 k
|
Communicate to external providers the requirements | Regarding the need to prevent the use of counterfeit parts, cf. sub-clause 8.1.4 | |
| 291 |
8.4.3 k
|
Communicate to external providers the requirements | Regarding the need to notify the organization of changes to processes, products or services, including changes of their external providers or location of manufacture and obtain the organization's approval | |
| 292 |
8.4.3 k
|
Communicate to external providers the requirements | Regarding the need to flow down to external providers applicable requirements including customer requirements | |
| 293 |
8.4.3 k
|
Communicate to external providers the requirements | Regarding the need to provide test specimens for design approval, inspection/verification, investigation or auditing | |
| 294 |
8.4.3 k
|
Communicate to external providers the requirements | Regarding the need to retain documented information, including retention periods and disposition requirements | |
| 295 |
8.4.3 l
|
Communicate to external providers the requirements | Regarding the right of access by the company, their customer and regulatory authorities to the applicable areas of facilities and to applicable documented information at any level of the supply chain | |
| 296 |
8.4.3 m
|
Communicate to external providers the requirements | Regarding the assurance that persons are made aware of their contribution to product or service conformity | |
| 297 |
8.4.3 m
|
Communicate to external providers the requirements | Regarding the assurance that persons are made aware of their contribution to product safety | |
| 298 |
8.4.3 m
|
Communicate to external providers the requirements | Regarding the assurance that persons are made aware of the importance of ethical behavior | |
| 8.5 |
Production and service provision
|
|||
| 8.5.1 |
Control of production and service provision
|
|||
| 299 | 8.5.1 | Apply controlled conditions of production and service provision | Including delivery and post-delivery activities | |
| 300 | 8.5.1 a 1 | Save documented information of specifications of products and services and the expected activities | Cf. sub-clause 7.5 | |
| 301 | 8.5.1 a 2 | Save the documented information of results to be achieved | Cf. sub-clause 7.5. "A quality control that does not show results is no control". Kaoru Ishikawa | |
| 302 | 8.5.1 b | Include in the controlled conditions the inspection resources | Cf. sub-clause 7.1.5 | |
| 303 | 8.5.1 c | Include in the controlled conditions the inspection activities | To verify that the appropriate stages of the processes' outputs meet the criteria | |
| 304 |
8.5.1 c 1
|
Ensure that documented information for monitoring and measurement activity includes criteria for acceptance or rejection |
Cf. sub-clause 7.5 | |
| 305 |
8.5.1 c 1
|
Ensure that documented information for monitoring and measurement activity includes stages where the verification operations are to be performed | Cf. sub-clause 7.5 | |
| 306 |
8.5.1 c 1
|
Ensure that documented information for monitoring and measurement activity includes measurement results to be obtained | At a minimum an indication of acceptance or rejection | |
| 307 |
8.5.1 c 1
|
Ensure that documented information for monitoring and measurement activity includes any specific monitoring and measurement equipment required | And instruction associated with their use | |
| 308 |
8.5.1 c 2
|
Ensure that when sampling is used as a means of product acceptance, the sampling plan is justified on the basis of recognized statistical principles and appropriate for use | Match the sampling plan to the criticality of the product and the process capability | |
| 309 | 8.5.1 d | Include in the controlled conditions adequate infrastructure and environment | Cf. sub-clauses 7.1.3 et 7.1.4 | |
| 310 | 8.5.1 e | Include in the controlled conditions the staff competence | Including the necessary qualification, cf. sub-clause 7.2 | |
| 311 | 8.5.1 f | Include in the controlled conditions the validation of the ability of a process to achieve the expected results | Only in the case when the outputs cannot be checked a posteriori | |
| 312 | 8.5.1 g | Include in the controlled conditions the actions to prevent human error | Use tools such as Poka-Yoke | |
|
313 |
8.5.1 h | Include in the controlled conditions the activities of release, delivery and post-delivery | Cf. sub-clause 8.6 and sub-clause 8.5.5 | |
| 314 |
8.5.1 i
|
Include in the controlled conditions the establishment of criteria for workmanship | Such as written standards, representative samples, illustrations | |
| 315 |
8.5.1 j
|
Include in the controlled conditions the accountability for all products during production | Such as parts quantity, split orders, nonconforming product | |
| 316 |
8.5.1 k
|
Include in the controlled conditions the control and monitoring of identified critical items in accordance with established processes | Including key characteristics | |
| 317 |
8.5.1 l
|
Include in the controlled conditions the determination of methods to measure variable data | Such as tooling, on-machine probing, inspection equipment | |
| 318 |
8.5.1 m
|
Include in the controlled conditions the identification of in-process inspection/verification points | When adequate verification of conformity cannot be performed at later stages | |
| 319 |
8.5.1 n
|
Include in the controlled conditions the availability of evidence that all production and inspection/verification operations have been completed as planned | Or as otherwise documented and authorized | |
| 320 |
8.5.1 o
|
Include in the controlled conditions the provision for the prevention, detection and removal of foreign objects | Cf. sub-clause 8.1.3 | |
| 321 |
8.5.1 p
|
Include in the controlled conditions the control and monitoring of utilities and supplies to the extent they affect conformity of product requirements | Such as water, compressed air, electricity, chemical products | |
| 322 |
8.5.1 q
|
Include in the controlled conditions the identification and recording of products released for subsequent production use to allow recall and replacement if it is later found that the product does not meet requirements | Pending completion of all required measuring and monitoring activities | |
| 8.5.1.1 |
Control of equipment, tools and software programs
|
|||
| 323 |
8.5.1.1
|
Validate and maintain equipment, tools and software programs used to automate, control, monitor or measure production processes |
Prior to release for production | |
| 324 |
8.5.1.1
|
Define storage requirements for production equipment and tooling in storage |
Including any necessary periodic preservation or condition checks | |
| 8.5.1.2 |
Validation and control of special processes
|
|||
| 325 |
8.5.1.2 a |
Establish, as applicable, arrangements for definition of criteria for the review and approval of the processes |
For processes where the resulting output cannot be verified by subsequent monitoring or measurement | |
| 326 |
8.5.1.2 b
|
Establish, as applicable, arrangements for determination of conditions to maintain the approval | For processes where the resulting output cannot be verified by subsequent monitoring or measurement | |
| 327 |
8.5.1.2 c
|
Establish, as applicable, arrangements for approval of facilities and equipment | For processes where the resulting output cannot be verified by subsequent monitoring or measurement | |
| 328 |
8.5.1.2 d
|
Establish, as applicable, arrangements for qualification of persons | For processes where the resulting output cannot be verified by subsequent monitoring or measurement | |
| 329 |
8.5.1.2 e
|
Establish, as applicable, arrangements for use of specific methods and procedures for implementation and monitoring the processes | For processes where the resulting output cannot be verified by subsequent monitoring or measurement | |
| 330 |
8.5.1.2 f
|
Establish, as applicable, arrangements for requirements for documented information to be retained | For processes where the resulting output cannot be verified by subsequent monitoring or measurement. Cf. sub-clause 7.5 | |
| 8.5.1.3 |
Production process verification
|
|||
| 331 |
8.5.1.3
|
Implement production process verification activities to ensure the production process is able to produce products that meet requirements |
Such as risk assessment, capacity studies, capability studies, control plans. These activities are "First article inspection", cf. AS 9102 | |
| 332 |
8.5.1.3
|
Use a representative item from the first production run of a new part or assembly |
In order to verify that the production processes, production documentation and tooling are able to produce parts and assemblies that meet requirements | |
| 333 |
8.5.1.3
|
Repeat this activity when changes occur that invalidate the original results |
Such as engineering, production process and tooling changes | |
| 334 |
8.5.1.3
|
Retain documented information on the results of production process verification |
Cf. sub-clause 7.5 | |
| 8.5.2 |
Identification and traceability
|
|||
| 335 | 8.5.2 | Use appropriate means to control the unique identification of process outputs | In order to ensure the conformity of products and services when needed | |
| 336 |
8.5.2
|
Maintain the identification of the configuration of the products and services |
In order to identify any differences between the actual and the required configuration | |
| 337 | 8.5.2 | Inspect processes throughout the production and service provision | In order to identify the status of process outputs | |
| 338 |
8.5.2
|
Establish controls for the media when acceptance authority media is used |
Such as stamps, electronic signatures, passwords | |
| 339 | 8.5.2 | Control the traceability of process outputs | When traceability is a requirement, the unique identification is used | |
| 340 | 8.5.2 | Retain the documented information on traceability | Cf. sub-clause 7.5. When traceability is a requirement, the unique identification of outputs is used | |
| 8.5.3 |
Property belonging to customers or external providers
|
|||
| 341 | 8.5.3 | Exercise care with property owned by customer or external provider | During its use or protection | |
| 342 | 8.5.3 | Identify, check, protect, monitor and safeguard customer or external provider property | When used or incorporated with updated labels | |
| 343 | 8.5.3 | Notify the customer or external provider when his property has been damaged or lost and retain the documented information on the situation | Following incorrect or improper use. Cf. sub-clause 7.5. | |
| 8.5.4 |
Preservation
|
|||
| 344 | 8.5.4 | Preserve the process outputs throughout production and service provision activities | Some examples of preservation methods: identification, packaging, handling, storage, transport, protection | |
| 345 |
8.5.4 a
|
Include in preservation of outputs provisions for cleaning |
When applicable in accordance with specifications and applicable statutory and regulatory requirements | |
| 346 |
8.5.4 b
|
Include in preservation of outputs provisions for prevention, detection and removal of foreign objects | When applicable in accordance with specifications and applicable statutory and regulatory requirements | |
| 347 |
8.5.4 c
|
Include in preservation of outputs provisions for special handling and storage for sensitive products | When applicable in accordance with specifications and applicable statutory and regulatory requirements | |
| 348 |
8.5.4 d
|
Include in preservation of outputs provisions for marking and labeling, including safety warnings and cautions | When applicable in accordance with specifications and applicable statutory and regulatory requirements | |
| 349 |
8.5.4 e
|
Include in preservation of outputs provisions for shelf life control and stock rotation | When applicable in accordance with specifications and applicable statutory and regulatory requirements | |
| 350 |
8.5.4 f
|
Include in preservation of outputs provisions for special handling and storage for hazardous materials | When applicable in accordance with specifications and applicable statutory and regulatory requirements | |
| 8.5.5 |
Post-delivery activities
|
|||
| 351 | 8.5.5 | Meet the requirements for post-delivery activities | Examples of post-delivery activities: exchange new product, maintenance, recycling, final disposal | |
| 352 | 8.5.5 a | Take into account statutory and regulatory requirements | Cf. sub-clause 4.2 | |
| 353 | 8.5.5 b | Take into account negative impacts related to products and services | These are consequences of potential risks | |
| 354 | 8.5.5 c | Take into account the nature, the intended use and lifetime of products and services | When the extent of post-delivery activities has been clarified | |
| 355 | 8.5.5 d | Take into account the requirements of the stakeholders | And customers especially | |
| 356 | 8.5.5 e | Take into account customer feedback | From stakeholders, when the extent of post-delivery activities has been clarified | |
| 357 |
8.5.5 f
|
Take into account collection and analysis of in-service data |
Such as performance, reliability, lessons learned | |
| 358 |
8.5.5 g
|
Take into account control, updating and provision of technical documentation | Such as product use, maintenance, repair and overhaul | |
| 359 |
8.5.5 h
|
Take into account controls required for work undertaken external to the organization | Such as off-site work | |
| 360 |
8.5.5 i
|
Take into account product/customer support | Such as queries, training, warranties, maintenance, replacement parts, resources, obsolescence | |
| 361 |
8.5.5
|
Take appropriate action when problems are detected after delivery |
Including investigation and reporting | |
| 8.5.6 |
Control of changes
|
|||
| 362 | 8.5.6 | Review and control unplanned changes | Cf. sub-clause 6.3 (planned changes) | |
| 363 |
8.5.6
|
Identify persons authorized to approve production and service provision changes |
Such as changes affecting processes, production equipment, tools or software programs | |
| 364 | 8.5.6 | Retain the documented information on unplanned changes | Cf. sub-clause 7.5. Include the results of reviews, the authorization of changes and actions implemented | |
| 8.6 |
Release of products and services
|
|||
| 365 | 8.6 | Check products and services with activities at appropriate stages | "Inspection does not improve quality, nor guarantee quality". Edwards Deming | |
| 366 | 8.6 | Release products and services after verification of conformity | Unless written approval (concession) by a competent authority or client | |
| 367 | 8.6 | Retain the documented information on the release of products and services | Cf. sub-clause 7.5. | |
| 368 | 8.6 a | Include in the documented information evidence of conformity | These are the results of inspections compared to the acceptance criteria | |
| 369 | 8.6 b | Include in the documented information the traceability of products and services | Including the person having authorized the release | |
| 370 |
8.6
|
Ensure that retained documented information provides evidence that the products and services meet the defined requirements |
When required to demonstrate product qualification | |
| 371 |
8.6
|
Ensure that all documented information required to accompany the products and services are present at delivery |
Cf. sub-clause 7.5 | |
| 8.7 |
Control of nonconforming outputs
|
|||
| 372 | 8.7.1 | Identify and treat nonconforming process, products and services outputs | Marking and isolation to prevent unintended use or mixing with conforming outputs | |
| 373 | 8.7.1 | Carry out corrective actions commensurate to impacts | Including after delivery. Cf. sub-clause 10.2 | |
| 374 | 8.7.1 | Carry out corrective actions on post-delivery activities | Cf. sub-clause 8.5.5 | |
| 375 |
8.7.1
|
Maintain documented information on the nonconformity control process including the provisions for defining the responsibility and authority for the review and disposition of nonconforming outputs |
Including the process for approving persons making these decisions | |
| 376 |
8.7.1
|
Maintain documented information on the nonconformity control process including the provisions for taking necessary actions | In order to limit the effect of nonconformity on other processes, products or services | |
| 377 |
8.7.1
|
Maintain documented information on the nonconformity control process including the provisions for timely reporting of nonconformities affecting delivered products and services | To the customer and to relevant stakeholders such as external providers, distributors and regulatory authorities | |
| 378 |
8.7.1
|
Maintain documented information on the nonconformity control process including the provisions for defining corrective actions for nonconforming products and services detected after delivery | Corrective actions as appropriate to their impacts. Cf. sub-clause 10.2 | |
| 379 | 8.7.1 a | Handle nonconforming outputs with corrections | Repeat work, retouching, repair, recycling | |
| 380 | 8.7.1 b | Handle nonconforming outputs by segregation | Including customer returns or products and services not released | |
| 381 | 8.7.1 c | Inform the customer | Cf. sub-clause 7.4 | |
| 382 | 8.7.1 d | Handle nonconforming outputs by asking authorization | To use-as-is (acceptance under concession), by a relevant authority, and when applicable, by the customer | |
| 383 |
8.7.1
|
Implement dispositions of use-as-is or repair for the acceptance of nonconforming products only after approval by an authorized representative |
The representative is authorized by the organization responsible for design or by persons having delegated authority | |
| 384 |
8.7.1
|
Implement dispositions of use-as-is or repair for the acceptance of nonconforming products only after authorization by the customer | If the nonconformity results in a departure from the contract requirements | |
| 385 |
8.7.1
|
Conspicuously and permanently mark product dispositioned for scrap until physically rendered unusable |
Or isolate it in a safe place | |
| 386 |
8.7.1
|
Inspect counterfeit, or suspected counterfeit parts |
In order to prevent reentry into the supply chain | |
| 387 | 8.7.1 | Check conformity after any correction | After any correction go through the normal flow | |
| 388 | 8.7.2 a | Retain the documented information on the description of nonconformities | Cf. sub-clause 7.5. | |
| 389 | 8.7.2 b | Retain the documented information on implemented actions | Cf. sub-clause 7.5. | |
| 390 | 8.7.2 c | Retain the documented information on approved concessions | Cf. sub-clause 7.5. | |
| 391 | 8.7.2 d | Retain the documented information on the person having decided the handling of the nonconformities | Cf. sub-clause 7.5 | |
| 9 |
Performance evaluation
|
Check | ||
| 9.1 |
Monitoring, measurement, analysis and evaluation
|
|||
| 9.1.1 |
General
|
|||
| 392 | 9.1.1 a | Determine what is necessary to inspect | "If you can’t measure it, you can’t manage it". Peter Drucker | |
| 393 | 9.1.1 b | Determine the methods for inspection, analysis and evaluation | In order to ensure valid results | |
| 394 | 9.1.1 c | Determine when to inspect | At key stages (essential) or upon the customer's request | |
| 395 | 9.1.1 d | Determine when to analyze and evaluate inspection results | When that brings added value | |
| 396 | 9.1.1 | Evaluate the performance and effectiveness of the QMS | In order to ensure that specified requirements are met | |
| 397 | 9.1.1 | Retain the documented information on the inspection results | Cf. sub-clause 7.5 | |
| 9.1.2 |
Customer satisfaction
|
|||
| 398 | 9.1.2 | Regularly monitor customer perception about their level of satisfaction | "The only measure of quality is customer satisfaction" | |
| 399 | 9.1.2 | Determine methods for obtaining and using customer information | Satisfaction surveys, claims, customer returns, recommendations | |
| 400 |
9.1.2
|
Include in the information to be monitored and used for the evaluation of customer satisfaction product and service conformity, on-time delivery performance and customer complaints |
And corrective action request | |
| 401 |
9.1.2
|
Develop and implement plans that address deficiencies identified and assess the effectiveness of the results |
In order to improve customer satisfaction | |
| 9.1.3 |
Analysis and evaluation
|
|||
| 402 | 9.1.3 | Analyze and evaluate inspection data | "Get the facts, analyze them and then do what seems right". Robert Waterman | |
| 403 | 9.1.3 a | Use analysis results | In order to evaluate how requirements are met. Cf. sub-clause 4.2 | |
| 404 | 9.1.3 b | Use analysis results | In order to evaluate the level of customer satisfaction. Cf. sub-clause 9.1.2 | |
| 405 | 9.1.3 c | Use analysis results | In order to evaluate the performance and effectiveness of the QMS | |
| 406 | 9.1.3 d | Use analysis results | In order to evaluate the effectiveness of planning. Cf. sub-clause 8.1 | |
| 407 | 9.1.3 e | Use analysis results | In order to evaluate the effectiveness of actions implemented to address risks and opportunities. Cf. sub-clause 6.1 | |
| 408 | 9.1.3 f | Use analysis results | In order to evaluate the performance of external providers. Cf. sub-clause 8.4 | |
| 409 | 9.1.3 g | Use analysis results | In order to evaluate the improvement opportunities of the QMS. Cf. sub-clause 10.3 | |
| 9.2 |
Internal audit
|
|||
| 410 | 9.2.1 a 1 | Conduct regularly planned internal audits | In order to determine whether the QMS meets internal company requirements. Cf. ISO 19011 | |
| 411 | 9.2.1 a 2 | Conduct regularly planned internal audits | In order to determine whether the QMS meets requirements of the ISO 9001 standard | |
| 412 | 9.2.1 b | Conduct regularly planned internal audits | In order to determine whether the QMS is effectively implemented and maintained | |
| 413 | 9.2.2 a | Plan, establish, implement and update an audit program | Include the frequency, methods, responsibilities, planning requirements (audit program) and reporting requirements (audit report) | |
| 414 | 9.2.2 a | Take into account in the audit program essential points | Essentials points :
|
|
| 415 | 9.2.2 b | Define the scope and audit criteria | Limit the area to be audited; use specific and known by the auditee criteria | |
| 416 | 9.2.2 c | Select auditors | Do not audit your department. "No one is judge in his own case". Latin proverb | |
| 417 | 9.2.2 d | Communicate audit results to management concerned | Cf. sub-clause 7.4 | |
| 418 | 9.2.2 e | Undertake a correction quickly and corrective actions if necessary | Cf. sub-clause 10.2 | |
| 419 | 9.2.2 f | Retain the documented information on the audit program and the audit reports | Cf. sub-clause 7.5 | |
| 9.3 |
Management review
|
|||
| 9.3.1 |
General
|
|||
| 420 | 9.3.1 | Proceed at least once a year to review the QMS | In order to confirm that it is still relevant, appropriate and effective. "No system is perfect" | |
| 9.3.2 |
Management review inputs
|
|
||
| 421 | 9.3.2 a | Plan and carry out the management review | Regarding the status of actions of the previous review | |
| 422 | 9.3.2 b | Carry out the management review taking into account the changes of external and internal issues for the QMS | Including strategic direction | |
| 423 | 9.3.2 c 1 | Take into account the information on the performance of the QMS and trends | Customer satisfaction, feedback. Cf. sub-clauses 8.7 et 10.2 | |
| 424 | 9.3.2 c 2 | Take into account the information on the performance of the QMS and trends | The achievement of quality objectives, cf. sub-clause 6.2 | |
| 425 | 9.3.2 c 3 | Take into account the information on the performance of the QMS and trends | Process performance and conformity of outputs. Cf. sub-clause 9.1 | |
| 426 | 9.3.2 c 4 | Take into account the information on the performance of the QMS and trends | Nonconformities and corrective actions. Cf. sub-clause 10.2 | |
| 427 | 9.3.2 c 5 | Take into account the information on the performance of the QMS and trends | Inspection results. Cf. sub-clause 9.1 | |
| 428 | 9.3.2 c 6 | Take into account the information on the performance of the QMS and trends | Audit results. Cf. sub-clause 9.2 | |
| 429 | 9.3.2 c 7 | Take into account the information on the performance of the QMS and trends | Performance of external providers. Cf. sub-clause 8.4 | |
| 430 |
9.3.2.c 8
|
Take into account the information on the performance of the QMS and trends |
On-time delivery performance. Cf. sub-clause 8.1 | |
| 431 | 9.3.2 d | Take into account resources | Availability of resources. Cf. sub-clause 7.1 | |
| 432 | 9.3.2 e | Take into account the effectiveness of actions | Implemented to address risks and opportunities. Cf. sub-clause 6.1 | |
| 433 | 9.3.2 f | Take into account improvement opportunities | Continual improvement. Cf. sub-clause 10.3 | |
| 9.3.3 |
Management review outputs
|
|||
| 434 | 9.3.3 a | Include decisions regarding opportunities for continual improvement in the outputs of the management review | Cf. sub-clause 10.3 | |
| 435 | 9.3.3 b | Include decisions regarding eventual changes to the QMS in the outputs of the management review | Cf. sub-clause 6.3 | |
| 436 | 9.3.3 c | Include decisions regarding new resource needs in the outputs of the management review | Cf. sub-clause 7.1 | |
| 437 |
9.3.3 d
|
Include decisions regarding risks identified in the outputs of the management review |
Cf. sub-clause 8.1.1 | |
| 438 | 9.3.3 | Retain the documented information on outputs of the review of management | Cf. sub-clause 7.5 | |
| 10 |
Improvement
|
Act | ||
| 10.1 |
General
|
|||
| 439 | 10.1 | Find improvement opportunities and implement necessary actions | In order to enhance customer satisfaction. "Where there is a problem, there is potential for improvement". Masaaki Imai | |
| 440 | 10.1 a | Improve products and services | Support innovation. In order to better meet current requirements and anticipate future requirements | |
| 441 | 10.1 b | Reduce negative impacts | Implementing corrective actions and global prevention (efficient QMS) | |
| 442 | 10.1 c | Improve the results of the QMS | To achieve the objectives of the QMS regarding performance | |
| 10.2 |
Nonconformity and corrective action
|
|||
| 443 | 10.2.1 a 1 | React to the nonconformity | In order to reduce costs. Including all claims by processing, controlling, correcting. "One of the best ways to measure quality is to calculate the price of nonconformities". Philip Crosby | |
| 444 | 10.2.1 a 2 | Take into account consequences | Think risk-based approach | |
| 445 | 10.2.1 b 1 | Examine the nonconformity | And if necessary decide to carry out a corrective action | |
| 446 | 10.2.1 b 2 | Investigate root causes | So that the nonconformity does not happen again | |
| 447 | 10.2.1 b 3 | Search for similar nonconformities | In order to apply the same recipe (why reinvent the wheel?) | |
| 448 | 10.2.1 c | Implement the necessary corrective actions | In order to treat the nonconformity | |
| 449 | 10.2.1 d | Review the effectiveness of any implemented corrective action | In order to check whether the action is finalized | |
| 450 | 10.2.1 e | Update risks and opportunities | If necessary | |
| 451 | 10.2.1 f | Make changes to the QMS | If necessary | |
| 452 |
10.2.1 g
|
Flow down corrective action requirements to an external provider |
When it is determined that the external provider is responsible for the nonconformity | |
| 453 |
10.2.1 h
|
Take specific actions when timely corrective actions are not achieved |
And actions were not effective | |
| 454 | 10.2.1 | Respond proportionally to nonconformities consequences | Do not make too much quality | |
| 455 |
10.2.1
|
Maintain documented information that defines the nonconformity and corrective action processes |
Cf. sub-clause 7.5 | |
| 456 | 10.2.2 a | Retain documented information on the nature of nonconformities | Cf. sub-clause 7.5 | |
| 457 | 10.2.2 b | Retain documented information on results of implemented actions | Cf. sub-clause 7.5 | |
| 10.3 |
Continual improvement
|
|||
| 458 | 10.3 | Improve continually the performance of the QMS | In order to find improvement opportunities | |
| 459 | 10.3 | Take into account the outputs of the analysis, evaluation and management review | Cf. sub-clause 9.1.3 and sub-clause 9.3 | |
| 460 |
10.3
|
Monitor the implementation of improvement activities |
And evaluate the effectiveness of the results | |
|
|
||||




