ISO 22716 requirements cosmetics Good Manufacturing Practices version 2007
20/10/2023
Quiz requirements ISO 22716 version 2007 You want to familiarize yourself with the structure of the standard, identify and understand the requirements of ISO 22716 version 2007, then it's up to you to play!
The "ISO 22716 Requirements version 2007" quiz will help you understand the main requirements of the standard.
The questions (
Do not think you can finish this quiz in less than an hour, or even two hours, unless of course you are a little genius!
Some news about ISO 22716 version 2007
The course T 23v07 ISO 22716 readiness version 2007 and its free demo without registration
The course T 43v07 ISO 22716 internal audit version 2007 and its free demo without registration
The T 73v07 training package ISO 22716 readiness and internal audit version 2018
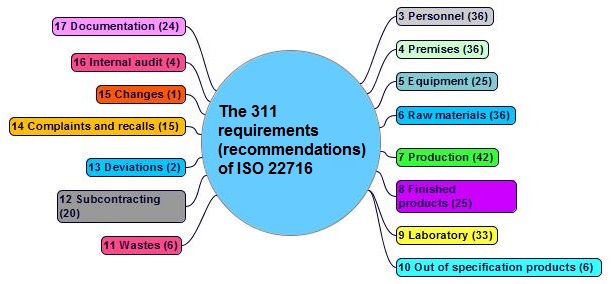
The 331 ISO 22716
|
No
|
Clause
|
PDCA cycle
|
Requirement No
|
Quantity
|
| 3 | Personnel | Plan | 1 ÷ 36 | 36 |
|
4
|
Premises | Plan | 37 ÷ 72 | 36 |
|
5
|
Equipment | Plan |
73 ÷ 97
|
25
|
|
6
|
Raw materials | Plan |
98 ÷ 133
|
36
|
|
7
|
Production | Do |
134 ÷ 176
|
42
|
|
8
|
Finished products | Do |
177 ÷ 200
|
25
|
|
9
|
Laboratory | Do |
201 ÷ 233
|
33
|
|
10
|
Nonconforming products | Check |
234 ÷ 239
|
6
|
| 11 | Wastes | Act | 240 ÷ 245 | 6 |
| 12 | Subcontracting | Do | 246 ÷ 265 | 20 |
| 13 | Deviations | Check | 266 ÷ 267 | 2 |
| 14 | Complaints and recalls | Act | 268 ÷ 282 | 15 |
| 15 | Change controls | Do | 283 | 1 |
| 16 | Internal audit | Check | 284 ÷ 287 | 4 |
| 17 | Documentation | Do | 288 ÷ 311 | 24 |
|
Total
|
331
|
|||
ISO 22716 requirements version 2007
.jpg)
The Deming PDCA cycle
Note. Any advice (recommendation, tip, wish, statement) in the standard begins with "The organization should...". For simplicity's sake we present the requirements directly, starting with the verb, as in the end we are dealing with requirements
|
ISO 22716 version 2007 - Requirements (recommendations) and comments
|
||||
|
No
|
clause, paragraph
|
Requirement (recommendation)
|
Comments, PDCA, links
|
|
|
3
|
Personnel
|
|||
|
|
Principle
|
|||
|
1
|
3.1
|
Have appropriate training to produce, control and store products of a defined quality |
For persons involved in the implementation of the activities described in the Good Manufacturing Practices Guidelines (ISO 22716), cf. § 3.4 |
|
|
Organization
|
|
|||
|
|
Organization chart
|
|
||
|
2
|
3.2.1.1
|
Define the organization chart |
In order to understand the organization and functioning of the company's staff. The organization chart may be functional, but another document must link the function and the person |
|
| 3 | 3.2.1.1 | Ensure that the organizational structure is appropriate | Concerning the size of the company and the diversity of its products | |
|
4
|
3.2.1.2
|
Ensure that staffing levels in the different areas of activity are adequate |
According to the diversity of its production | |
| 5 |
3.2.1.3
|
Demonstrate the independence of the quality units | In the organization chart | |
| 6 | 3.2.1.3 | Assume responsibilities for quality assurance and quality control |
By a single unit or by two separate units |
|
|
|
Number of people
|
|||
|
7
|
3.2.2
|
Have an adequate number of trained personnel |
With regards to the activities defined in the guidelines on Good Manufacturing Practices (ISO 22716), cf. § 3.4 | |
|
|
Key responsibilities
|
|||
|
|
3.3.1 |
Management responsibilities
|
|
|
|
8
|
3.3.1.1
|
Support the organization | By the top management of the company | |
|
9
|
3.3.1.2
|
Implement Good Manufacturing Practices | Responsibility of top management | |
|
10
|
3.3.1.2
|
Require the participation and commitment of personnel from all departments | And at all levels within the company | |
| 11 |
3.3.1.3
|
Define and communicate the areas in which authorized personnel have access | Responsibility of top management | |
|
|
3.3.2 |
Responsibilities of personnel
|
|
|
|
12
|
3.3.2 a
|
Know their position in the organization chart | For all personnel | |
| 13 | 3.3.2 b | Know their defined responsibilities and activities | For all personnel | |
|
14
|
3.3.2 c
|
Have access to and comply with documents relevant to their particular responsibility scope | For all personnel, cf. clause 17 | |
| 15 | 3.3.2 d | Follow personal hygiene requirements | For all personnel, cf. § 3.5 | |
| 16 | 3.3.2 e | Be encouraged to report irregularities and other nonconformities which may occur in the field | For all personnel | |
| 17 | 3.3.2 f |
Have appropriate training and knowledge to perform the assigned responsibilities and activities |
For all personnel, cf. § 3.4 | |
|
Training
|
|
|||
|
|
3.4.1
|
Training and skills
|
||
|
18
|
3.4.1
|
Have skills based on adequate training and experience acquired both appropriate to their responsibilities and activities |
For personnel involved in production, control, storage and shipment | |
|
|
3.4.2
|
Training and Good Manufacturing Practices
|
|
|
|
19
|
3.4.2.1
|
Provide for all personnel appropriate Good Manufacturing Practices training |
In relation to the guidelines of ISO 22716 and specific activities such as the use of a scale and control equipment |
|
|
20
|
3.4.2.2
|
Identify training needs of all personnel | Regardless of level or seniority in the company | |
|
21
|
3.4.2.2
|
Develop a training program | And implemented | |
|
22
|
3.4.2.3
|
Design training courses tailored to be appropriate to people's jobs and responsibilities |
Considering the expertise and experience of the respective personnel | |
|
23
|
3.4.2.4
|
Design and execute training courses in-house | By the company itself or with the help of external organizations | |
|
24
|
3.4.2.5
|
Regard training as a constant and on-going process | Process that is subject to regular updates | |
|
|
3.4.3
|
Newly recruited personnel
|
||
|
25
|
3.4.3
|
Receive appropriate training for the tasks of newly recruited personnel |
Besides basic training on the theory and practice of Good Manufacturing Practices | |
|
|
3.4.4
|
Personnel training evaluations
|
||
|
26
|
3.4.4
|
Evaluate knowledge accumulated by personnel |
During or after training. Hot, at the end of training and cold, two to three months later. Evaluate the personnel and not the training |
|
|
Personnel hygiene and health
|
|
|||
|
|
Personnel hygiene
|
|||
| 27 |
3.5.1.1
|
Establish and adapt hygiene programs | To the needs of the plant | |
| 28 | 3.5.1.1 |
Ensure that the requirements of hygiene programs are understood and followed |
By every person whose activities take them into production, control and storage areas |
|
|
29
|
3.5.1.2
|
Be instructed to use hand washing facilities |
For all personnel | |
|
30
|
3.5.1.3
|
Wear suitable clothing and protective garments for every person entering production, control and storage areas |
In order to avoid contamination of cosmetic products. Appropriate clothing is common (blouse, shoes), while protective clothing is specific (mask, gloves, cap) when personnel are in direct contact with the raw material or product |
|
|
31
|
3.5.1.4
|
Avoid eating, drinking, chewing and smoking in production, control and storage areas |
Or even holding food, beverages, tobacco or medicines in these areas |
|
|
32
|
3.5.1.5
|
Prohibit unhygienic practice in production, control and storage areas |
Or in any other area where the product might be adversely affected |
|
|
|
Personnel health
|
|||
|
33
|
3.5.2
|
Take steps to ensure, as far as possible, that any person suffering from an apparent illness or with uncovered wounds is excluded from direct contact with cosmetic products |
Until the condition is corrected or determined by medical personnel that the quality of cosmetic products will not be compromised |
|
|
3.6
|
Visitors and untrained personnel
|
|||
| 34 | 3.6 |
Do not take visitors or untrained personnel into production, control and storage areas |
If reasonably possible | |
| 35 | 3.6 |
Inform in advance about personal hygiene and the prescribed protective clothing |
When this is unavoidable | |
| 36 | 3.6 |
Supervise closely visitors and untrained personnel |
In the event that these persons still have to go to production, control and storage areas | |
|
Premises
|
||||
|
Principle
|
||||
|
37
|
4.1.1 a
|
Ensure protection of the product | By means of suitably located, designed, constructed and utilized premises | |
| 38 | 4.1.1 b | Allow efficient cleaning, sanitizing, if necessary, and maintenance | By means of suitably located, designed, constructed and utilized premises | |
| 39 | 4.1.1 c | Minimize the risk of mix-up | Of products, raw materials and packaging materials by means of suitably located, designed, constructed and utilized premises | |
| 40 | 4.1.2 | Base decisions on the design of the premises |
Based on the type of cosmetic product produced, existing conditions, cleaning and, if necessary, sanitizing measures used |
|
|
4.2
|
Types of area
|
|
||
|
41
|
4.2
|
Provide separate areas |
For storage, production, quality control, ancillary, washing and toilets |
|
|
|
Space
|
|||
|
42
|
4.3
|
Provide sufficient space | To facilitate operations such as receipt, storage and production | |
|
Flow
|
|
|||
|
43
|
4.4
|
Define the flow of materials, products and personnel | In order to prevent mix-ups | |
|
|
Floors, walls, ceilings, windows
|
|
||
| 44 | 4.5.1 | Design or construct floors, walls, ceilings and windows in production areas | For ease of cleaning and, if necessary, sanitization | |
|
45
|
4.5.1
|
Keep floors, walls, ceilings and windows clean in production areas |
And in good repair | |
|
46
|
4.5.2
|
Do not open windows | Where ventilation is adequate, cf. § 4.8 | |
|
47
|
4.5.2
|
Protect properly windows with a screen |
When windows are opened | |
|
48
|
4.5.3
|
Allow for proper cleaning and maintenance |
For new construction of production areas | |
|
49
|
4.5.3
|
Include, if necessary, smooth surfaces | When design of new construction | |
|
50
|
4.5.3
|
Allow for resistance to corrosive cleaning and sanitizing agents |
For smooth surfaces when design of new construction | |
|
4.6
|
Washing and toilet facilities
|
|||
| 51 |
4.6
|
Provide for personnel adequate, clean, washing and toilets facilities |
Provide a room for hand washing | |
| 52 |
4.6
|
Differentiate toilet and washing facilities from production areas |
While remaining accessible | |
| 53 |
4.6
|
Provide adequate facilities | For showering and changing clothes | |
|
4.7
|
Lighting
|
|||
| 54 | 4.7.1 | Install adequate lighting in all areas | That is sufficient for operations | |
|
55
|
4.7.2
|
Install lighting in a manner to ensure containment of any debris | From potential breakage | |
|
56
|
4.7.2
|
Take measures, if necessary | To protect the product | |
|
|
Ventilation
|
|
||
| 57 | 4.8 | Provide adequate ventilation | For the intended production operations | |
| 58 | 4.8 | Take specific measures, if necessary | To protect the product | |
|
|
Pipework, drains and ducts
|
|||
|
59
|
4.9.1
|
Install pipework, drains and ducts |
In such a manner so that drip or condensation does not contaminate materials, products, surfaces and equipment |
|
|
60
|
4.9.2
|
Keep drains clean | And should not allow back flow | |
|
61
|
4.9.3 a
|
Take into consideration when designing |
Avoid exposed overhead roof beams, pipes and ducts |
|
|
62
|
4.9.3 b
|
Take into consideration when designing |
Exposed pipes should not touch walls, but be suspended from or supported by brackets, sufficiently separated to allow thorough cleaning |
|
|
63
|
4.9.3 c
|
Take into consideration when designing |
Specific measures should be taken to protect the product, if necessary |
|
|
|
Cleaning and sanitization
|
|||
| 64 | 4.10.1 | Maintain premises in a clean condition |
Cleaning is planned and carried out by qualified personnel |
|
| 65 | 4.10.2 | Carry out cleaning, and, if necessary, sanitization | To protect each cosmetic product | |
| 66 |
4.10.3
|
Use cleaning, and, if necessary, sanitizing agents | Specified and effective | |
| 67 |
4.10.4
|
Have cleaning and, if necessary, sanitization programs | Corresponding to specific needs of each area | |
|
4.11
|
Maintenance
|
|
||
|
68
|
4.11
|
Maintain premises in a good state of repair | Cf. the other paragraphs of clause 4 | |
|
|
Consumables
|
|||
|
69
|
4.12
|
Use appropriate consumables for production and storage premises | That do not affect the quality of the product | |
|
4.13
|
Pest control
|
|
||
|
70
|
4.13.1
|
Design, construct and maintain the premises in such a way as to limit the access of parasites |
Such as insects, birds, rodents, pests and other vermin | |
| 71 | 4.13.2 |
Establish a pest control protection program appropriate for the premises |
Inside the premises, set up physical traps and trap monitoring | |
|
72
|
4.13.3
|
Take measures to control the exterior of the premises |
In order to avoid attracting pests or providing shelter for them |
|
|
5
|
Equipment
|
|||
|
Principle
|
||||
| 73 | 5.1 |
Adapt the equipment for the intended use |
This applies to all equipment falling within the scope of the ISO 22716 cosmetic Good Manufacturing Practice guidelines |
|
| 74 | 5.1 |
Clean and, if necessary, sanitize, and maintain |
For all equipment | |
| 75 | 5.1 |
Take into account the application of the given appropriate principles |
For the use of automated systems | |
|
Equipment design
|
|
|||
|
76
|
5.2.1
|
Design production equipment that is easy to maintain |
To prevent contamination of the product | |
| 77 | 5.2.2 |
Protect bulk containers from contaminants |
Carried by air such as dust and moisture | |
|
78
|
5.2.3
|
Clean and, if necessary, sanitize transfer hoses and accessories used |
And kept dry and protected from dust, splash or other contamination |
|
|
79
|
5.2.4
|
Ensure that the materials used in the construction of equipment are compatible with cosmetic products |
And cleaning and sanitizing agents | |
|
Installation
|
||||
|
80
|
5.3.1
|
Provide that the design and installation of equipment ease its drainage |
In order to facilitate cleaning and sanitization |
|
|
81
|
5.3.2
|
Arrange equipment in such a way that the movement of materials, mobile equipment and personnel does not present any risk |
To quality | |
|
82
|
5.3.3
|
Provide reasonable access under, inside and around equipment |
For maintenance and cleaning | |
|
83
|
5.3.4
|
Facilitate the identification | Of major equipment | |
|
Calibration
|
||||
|
84
|
5.4.1
|
Calibrate regularly laboratory and production measuring instruments | For instruments that are important for the quality of the product | |
| 85 | 5.4.2 |
Identify properly and remove from service measuring instruments |
When results of calibration are out-of-acceptance criteria | |
| 86 |
5.4.3
|
Investigate when a calibration result is outside the acceptance criteria |
In order to determine whether this result has an impact on the quality of the product |
|
| 87 |
5.4.3
|
Take appropriate steps | Based on the results of the investigation | |
|
Cleaning and sanitization
|
||||
|
88
|
5.5.1
|
Be subject to an appropriate cleaning and, if necessary sanitization program |
For all equipment | |
|
89
|
5.5.2
|
Use cleaning and, if necessary sanitizing agents |
Specified and effective | |
|
90
|
5.5.3
|
Clean and, if necessary sanitize equipment used for continuous production or production of successive batches of the same product |
At appropriate intervals | |
|
Maintenance
|
||||
|
91
|
5.6.1
|
Maintain equipment | Regularly | |
| 92 | 5.6.2 |
Ensure that maintenance operations do not affect the quality of the product |
After each maintenance check that everything is compliant |
|
| 93 |
5.6.3
|
Identify defective equipment accordingly |
And excluded from use and isolated, if possible | |
|
|
Consumables
|
|
||
|
94
|
5.7
|
Ensure that consumables used for the equipment do not affect the quality of the product |
Consumables are specified and effective | |
|
|
Authorizations
|
|||
|
95
|
5.8
|
Use only accessible equipment or automated systems for production and control purposes |
By authorized personnel | |
| 96 | 5.8 |
Ensure that equipment or automated systems for production and control are only used by authorized personnel |
Training and certification, if required, cf. § 3.4 | |
|
|
Back-up systems
|
|
||
|
97
|
5.9
|
Ensure the availability of adequate alternative arrangements |
In case of failure or breakdown for systems which need to be operated |
|
|
6
|
Raw materials and packaging materials
|
|||
|
|
Principle
|
|
||
|
98
|
6.1
|
Purchase raw materials and packaging materials that meet the defined acceptance criteria |
And appropriate for the quality of finished products | |
|
Purchasing
|
||||
|
99
|
6.2 a
|
Base purchasing of raw materials and packaging materials on supplier evaluation |
And section of the supplier | |
| 100 | 6.2 b |
Base purchasing of raw materials and packaging materials on establishment of technical clauses |
Such as type of selection to be conducted, acceptance criteria, actions in the case of defect or modifications, transport conditions |
|
| 101 | 6.2 c |
Base purchasing of raw materials and packaging materials on setting of relations and exchanges between the company and the supplier |
Such as questionnaire, assistance and audits | |
|
Receipt
|
||||
|
102
|
6.3.1
|
Ensure that the purchase order, the delivery note and the materials delivered match |
Receipt instruction | |
|
103
|
6.3.2
|
Check visually the integrity of the shipping containers for raw materials |
And packaging materials | |
|
104
|
6.3.2
|
Perform additional checks, if necessary | Of transport data | |
|
Identification and status
|
||||
| 105 | 6.4.1 |
Label raw material containers and packaging materials |
In order to identify the material and the batch information | |
| 106 |
6.4.2
|
Put on hold for decision raw materials and packaging materials when they have defects |
That might affect product quality | |
| 107 | 6.4.3 |
Identify appropriately raw materials and packaging materials according to their status |
Such as accepted, rejected or quarantined. Other systems can replace this physical system of identification, if they ensure the same level of assurance |
|
| 108 |
6.4.4 a
|
Include in the information on the identification of raw materials and packaging materials |
Name of the product marked on the delivery note | |
| 109 | 6.4.4 b | Include in the information on the identification of raw materials and packaging materials |
Name of the product as given by the company, if different from the name given by the supplier or its code number |
|
| 110 |
6.4.4 c
|
Include in the information on the identification of raw materials and packaging materials | date or number of receipt, if appropriate | |
| 111 |
6.4.4 d
|
Include in the information on the identification of raw materials and packaging materials | Name of supplier | |
| 112 | 6.4.4 e | Include in the information on the identification of raw materials and packaging materials | Batch reference given by the supplier and the one given at receipt, if different | |
|
Release
|
||||
| 113 | 6.5.1 |
Set up a physical or alternative system |
In order to ensure that only released raw materials and packaging materials are used | |
|
114
|
6.5.2
|
Carry out the release of raw materials and packaging materials by authorized personnel |
Who is also responsible for quality | |
|
115
|
6.5.3
|
Accept raw materials and packaging materials on the basis of the supplier certificate of analysis |
Only if there are established technical requirements, experience and knowledge of the supplier and supplier audit |
|
| 116 | 6.5.3 | Accept raw materials and packaging materials on the basis of the supplier certificate of analysis |
If the supplier's methods have been agreed upon |
|
|
Storage
|
||||
| 117 | 6.6.1 |
Ensure that storage conditions are appropriate for each raw material |
And at each packaging material | |
| 118 | 6.6.2 |
Store and handle properly raw materials and packaging materials |
In relation to their characteristics such as not laying them on the ground |
|
| 119 | 6.6.3 |
Observe and monitor specific storage conditions |
Where appropriate | |
| 120 | 6.6.4 |
Ensure that containers of raw materials and packaging materials are closed |
And that they are not placed directly on the ground | |
| 121 | 6.6.5 |
Ensure that raw materials and packaging materials bear the same labeling as at origin |
When these materials are repacked | |
| 122 | 6.6.6 |
Store raw materials and packaging materials in their respective physical locations or by using any other system providing the same level of assurance |
When these materials are quarantined or rejected | |
| 123 | 6.6.7 |
Set up measures to ensure stock turnover |
Such as FIFO (Fist In, Fist Out) | |
| 124 | 6.6.7 | Ensure stock rotation |
The oldest released stock is used first |
|
| 125 | 6.6.8 | Perform periodic inventory | In order to ensure stock reliability | |
| 126 | 6.6.8 | Investigate any significant discrepancy | And take corrective action | |
|
Re-evaluation
|
||||
|
127
|
6.7
|
Set up an appropriate system to re-evaluate raw materials |
In order to determine their suitability for use, after a defined period of storage |
|
| 128 | 6.7 |
Set up a system to prevent the use of materials requiring re-evaluation |
If it is reasonably possible | |
|
Quality of water used in production
|
|
|||
| 129 | 6.8.1 |
Ensure that the water treatment system provides water of defined quality |
Cf. clause 9 | |
| 130 | 6.8.2 |
Check water quality through testing |
Or by monitoring of process parameters | |
| 131 | 6.8.3 |
Ensure that the water treatment system allows for sanitization |
Cf. clause 9 | |
| 132 | 6.8.4 |
Set up water treatment equipment so as to avoid stagnation |
And risks of contamination | |
| 133 | 6.8.5 |
Select materials for water treatment equipment |
In order to ensure that water quality is not affected | |
|
7
|
Production
|
D (Do) | ||
|
Principle
|
||||
| 134 | 7.1 |
Take measures to produce a finished product that meet the defined characteristics |
At each stage of manufacturing operations and packaging operations | |
|
|
Manufacturing operations
|
|
||
|
|
Availability of relevant documents
|
|
||
| 135 | 7.2.1.1 |
Ensure the availability of relevant documentation |
At each stage of manufacturing operations, cf. clause 17 | |
| 136 | 7.2.1.2 a |
Carry out manufacturing operations according to manufacturing documentation |
Including suitable equipment, cf. clause 17 | |
| 137 | 7.2.1.2 b | Carry out manufacturing operations according to manufacturing documentation | Including formula for the product, cf. clause 17 | |
| 138 | 7.2.1.2 c | Carry out manufacturing operations according to manufacturing documentation | Including list of all raw materials and batch numbers and quantities, cf. clause 17 | |
| 139 | 7.2.1.2 d | Carry out manufacturing operations according to manufacturing documentation |
Including manufacturing operations, such as addition of raw materials, temperatures, speeds, mixing times, sampling, cleaning and, if necessary, sanitizing of equipment, and bulk product transfer. All this information is part of the flow chart, cf. clause 17 |
|
|
|
7.2.2
|
Start-up checks
|
||
| 140 | 7.2.2 a |
Ensure that before starting any manufacturing operation |
All documentation relevant to the manufacturing operations is available | |
| 141 | 7.2.2 b | Ensure that before starting any manufacturing operation | All raw materials are available (and released) | |
| 142 | 7.2.2 c | Ensure that before starting any manufacturing operation | Suitable equipment is available for use, in working order, cleaned and, if necessary, sanitized | |
| 143 | 7.2.2 d | Ensure that before starting any manufacturing operation | Clearance of the area has been performed to avoid mixing with materials from previous operations | |
|
|
7.2.3
|
Assignment of batch number
|
|
|
| 144 | 7.2.3 | Assign a batch number | To each batch of manufactured bulk product | |
| 145 | 7.2.3 |
Associate the batch number of manufactured bulk product |
With the batch number that appears on the label | |
|
|
7.2.4
|
Identification of in-process operations
|
|
|
| 146 | 7.2.4.1 |
Measure or weigh all raw materials into clean and suitable containers, labeled with appropriate identification or directly into the equipment used for manufacturing |
In accordance with the formula | |
|
147
|
7.2.4.2
|
Be able to identify major equipment, raw material containers and bulk product containers |
At all times | |
| 148 | 7.2.4.3 a | Identify for bulk product containers | Name or identifying code | |
| 149 | 7.2.4.3 b | Identify for bulk product containers | Batch number | |
| 150 | 7.2.4.3 c | Identify for bulk product containers | Storage conditions when such information is critical to assure the quality of the product | |
|
|
In-process control
|
|||
|
151
|
7.2.5.1
|
Define in-process controls |
And acceptance criteria | |
| 152 | 7.2.5.2 | Perform in-process controls | According to a defined program | |
| 153 | 7.2.5.3 | Report any result outside the acceptance criteria | And appropriately investigated | |
|
|
7.2.6
|
Bulk product storage
|
|
|
| 154 | 7.2.6.1 | Store bulk product in suitable containers | And in defined areas, and under appropriate conditions | |
| 155 | 7.2.6.2 | Define the maximum bulk product storage duration | Of a bulk product | |
| 156 |
7.2.6.3
|
Re-evaluate the bulk product | When the maximum storage duration is reached | |
|
|
7.2.7
|
Re-stocking raw materials
|
|
|
| 157 | 7.2.7 |
Close and properly identify containers when raw materials remain unused after weighing |
And these materials are deemed acceptable to return to stock |
|
|
|
7.3 |
Packaging operations
|
|
|
| 7.3.1 |
Availability of relevant documents
|
|
||
| 158 |
7.3.1.1
|
Ensure availability of relevant documentation | At each stage of packaging operations, cf. clause 17 | |
|
159
|
7.3.1.2 a
|
Carry out packaging operations according to packaging documentation | Including suitable equipment, cf. clause 17 | |
|
160
|
7.3.1.2 b
|
Carry out packaging operations according to packaging documentation | Including list of packaging materials defined for the intended finished product, cf. clause 17 | |
|
161
|
7.3.1.2 c
|
Carry out packaging operations according to packaging documentation | Including packaging operations such as filling, closing, labeling, and coding, cf. clause 17 | |
|
|
7.3.2 |
Star-up checks
|
||
| 162 |
7.3.2 a
|
Ensure before the start of any packaging operation that the area has been cleared of materials |
In order to avoid mixing with materials from previous operations | |
|
163
|
7.3.2 b
|
Ensure before the start of any packaging operation that all documentation relevant to the packaging operation is available |
Cf. clause 17 | |
|
164
|
7.3.2 c
|
Ensure before the start of any packaging operation |
All packaging materials are available | |
|
165
|
7.3.2 d
|
Ensure before the start of any packaging operation that suitable equipment is available for use |
In working order, cleaned and, if necessary, sanitized | |
|
166
|
7.3.2 e
|
Ensure before the start of any packaging operation that any coding is defined |
In order to permit identification of the product | |
| 7.3.3 |
Assignment of batch number
|
|
||
| 167 |
7.3.3.1
|
Assign a batch number | To each unit of finished product | |
|
168
|
7.3.3.2
|
Associate the batch number of bulk product |
With the batch number that appears on the label of the bulk product | |
|
|
7.3.4 |
Packaging line identification
|
||
| 169 |
7.3.4
|
Ensure that the packaging line can be identified at any time with its name or identifying code |
Including the name or identification code of the finished product and the lot number |
|
| 7.3.5 |
Checks of on-line control equipment
|
|
||
|
170
|
7.3.5
|
Check regularly on-line control equipment, if used |
According to a defined program | |
| 7.3.6 |
In-process control
|
|
||
| 171 |
7.3.6.1
|
Define in-process controls | And their acceptance criteria | |
|
172
|
7.3.6.2
|
Perform in-process controls | According to a defined program | |
|
173
|
7.3.6.3
|
Report any result that is outside the acceptance criteria | And investigate appropriately | |
|
|
7.3.7 |
Re-stocking of packaging materials
|
|
|
| 174 |
7.3.7
|
Close and properly identify containers when packaging materials remain unused after packaging operations |
And these raw materials are intended to be returned to stock and are considered acceptable |
|
| 7.3.8 |
Identification and handling of work-in-process
|
|
||
| 175 |
7.3.8
|
Apply special measures when filling and labeling is not a continuous process |
Include segregation and identification in order to avoid mix-ups or mislabeling | |
|
8
|
Finished products
|
|||
|
|
Principle
|
|||
| 176 | 8.1 |
Ensure that the finished products meet the defined acceptance criteria |
The finished product (see definition 2.15) is a cosmetic product that cannot be ingested, inhaled, injected or implanted into the body |
|
| 177 | 8.1 |
Manage storage, shipment and returns |
In a manner so as to maintain the quality of finished products | |
|
|
8.2 |
Release
|
|
|
| 178 |
8.2.1
|
Control all finished products before being placed on the market |
In accordance with established test methods | |
| 179 |
8.2.1
|
Control all finished products before being placed on the market | In order to verify if they comply with acceptance criteria | |
| 180 |
8.2.2
|
Carry out product release | By the authorized personnel responsible for quality | |
|
|
Storage
|
|
||
| 181 |
8.3.1
|
Store finished products in defined areas under appropriate conditions |
For an appropriate length of time | |
| 182 |
8.3.1
|
Monitor finished products while stored |
If necessary | |
| 183 |
8.3.2
|
Permit organized storage | In storage areas | |
| 184 |
8.3.3
|
Store released, quarantined or rejected finished products in their respective physical locations |
Or by using any other system providing the same level of assurance | |
| 185 |
8.3.4 a
|
Ensure that the identification of finished product containers includes |
Name or identifying code | |
| 186 | 8.3.4 b | Ensure that the identification of finished product containers includes | Batch number | |
|
187
|
8.3.4 c
|
Ensure that the identification of finished product containers includes | storage conditions when such information is critical to assure the quality of the product | |
|
188
|
8.3.4 d
|
Ensure that the identification of finished product containers includes | Quantity | |
| 189 | 8.3.5 | Set up measures to ensure stock turnover | As FIFO, cf. clause 6.6 | |
| 190 |
8.3.5
|
Ensure stock rotation, except in special circumstances |
The oldest released stock is used first | |
|
191
|
8.3.6 a
|
Perform periodic inventory checks | In order to ensure inventory accuracy | |
| 192 |
8.3.6 b
|
Perform periodic inventory checks | In order to ensure that acceptance criteria are met | |
|
193
|
8.3.6
|
Investigate any significant discrepancy | If need be, set up a corrective action | |
|
|
8.4 |
Shipment
|
||
| 194 |
8.4
|
Take appropriate measures | In order to ensure the shipment of the defined finished product | |
|
195
|
8.4
|
Take precautions, when appropriate | In order to maintain the finished product quality | |
| 8.5 |
Returns
|
|
||
| 196 |
8.5.1
|
Identify returns in an appropriate way | And store returns in defined areas ("prison"). | |
|
197
|
8.5.2
|
Evaluate returns | Against established criteria to determine their disposition | |
|
198
|
8.5.3
|
Release returns | Before placing them on the market again | |
| 199 |
8.5.4
|
Establish measures | In order to distinguish any reprocessed return | |
| 200 |
8.5.4
|
Take measures |
In order to avoid the inadvertent redistribution of unreleased finished product |
|
|
Quality control laboratory
|
||||
|
|
Principle
|
|
||
| 201 | 9.1.1 |
Apply the principles described in ISO 22716 to the quality control laboratory |
Relating to personnel, cf. clause 3, to premises, cf. clause 4, to equipment, cf. clause 5, to subcontracting, cf. clause 12 and to documentation, cf. clause 17 | |
| 202 | 9.1.2 |
Carry out the relevant controls during sampling and testing by the laboratory |
So that materials are released for use |
|
| 203 |
9.1.2
|
Carry out the relevant controls during sampling and testing by the laboratory |
In order to release products for shipment only if their quality meets the required acceptance criteria |
|
|
|
Test methods
|
|||
| 204 |
9.2.1
|
Use all necessary test methods (by the quality control laboratory) |
In order to confirm that the product complies with acceptance criteria |
|
| 205 |
9.2.2
|
Perform controls on the basis of defined test methods |
Appropriate and available | |
|
|
Acceptance criteria
|
|
||
|
206
|
9.3
|
Establish acceptance criteria |
In order to specify the requirements to be met by raw materials and packaging materials, see clause 6, bulk products and finished products, cf. clause 8 |
|
| 9.4 |
Results
|
|
||
| 207 |
9.4
|
Review all results | In order to make a decision | |
|
208
|
9.4
|
Make a decision | Approval, rejection or pending | |
|
|
9.5 |
Out-of-specification results
|
|
|
| 209 |
9.5.1
|
Review out-of-specification results by authorized personnel |
And conduct a proper investigation to find the root causes | |
|
210
|
9.5.2
|
Provide sufficient justification | For any re-testing to be performed | |
|
211
|
9.5.3
|
Make a decision by authorized personnel, after the investigation |
Deviation, rejection or pending | |
|
|
9.6 |
Reagents, solutions, reference standards, culture media
|
||
| 212 |
9.6 a
|
Identify reagents, solutions, reference standards, culture media and others with clear information |
Name | |
| 213 |
9.6 b
|
Identify reagents, solutions, reference standards, culture media and others with clear information | Strength or concentration, when appropriate | |
| 214 | 9.6 c | Identify reagents, solutions, reference standards, culture media and others with clear information | Expiration date, when appropriate | |
| 215 | 9.6 d | Identify reagents, solutions, reference standards, culture media and others with clear information | Name or signature of the person who realized the preparation, when appropriate | |
| 216 |
9.6 e
|
Identify reagents, solutions, reference standards, culture media and others with clear information | Opening date | |
| 217 |
9.6 f
|
Identify reagents, solutions, reference standards, culture media and others with clear information | Storage conditions, when appropriate | |
|
|
Sampling
|
|
||
| 218 |
9.7.1
|
Perform sampling by authorized personnel | From laboratory or quality department | |
| 219 |
9.7.2 a
|
Define sampling in terms of | Sampling method | |
| 220 |
9.7.2 b
|
Define sampling in terms of | Equipment to be used | |
| 221 |
9.7.2 c
|
Define sampling in terms of | Amounts to be taken | |
| 222 |
9.7.2 d
|
Define sampling in terms of | Any precautions to be observed to avoid contamination or deterioration | |
| 223 | 9.7.2 e | Define sampling in terms of | Identification of sample | |
|
224
|
9.7.2 f
|
Define sampling in terms of | Frequency | |
| 225 |
9.7.3 a
|
Identify samples by | The name or identifying code | |
|
226
|
9.7.3 b
|
Identify samples by | The batch number | |
|
227
|
9.7.3 c
|
Identify samples by | The date of sampling | |
| 228 |
9.7.3 d
|
Identify samples by | The container from which the sample was taken | |
|
229
|
9.7.3 e
|
Identify samples by | The sampling point, if applicable | |
|
|
9.8 |
Retain sample
|
||
| 230 |
9.8.1
|
Retain samples of finished product in an appropriate manner | And in designed areas | |
|
231
|
9.8.2
|
Carry out analyses in accordance with local regulations |
The size of the samples of finished products should allow it | |
|
232
|
9.8.3
|
Keep retain samples of finished products in their primary package |
For an appropriate time under the recommended storage conditions | |
|
233
|
9.8.4
|
Retain samples of raw materials, if necessary | According to company practice or in accordance with local regulations | |
| 10 |
Treatment of product that is out of specification
|
|||
| 10.1 |
Rejected finished products, bulk products, raw materials and packaging materials
|
|
||
| 234 |
10.1.1
|
Perform investigations of rejected product or materials | By personnel authorized to do so | |
| 235 | 10.1.2 | Approve decisions to destroy or to reprocess | By the personnel responsible for quality | |
|
|
10.2 |
Reprocessed finished products and bulk products
|
|
|
| 236 |
10.2.1
|
Approve the reprocessing decision to obtain the defined quality for a batch that does not meet the defined acceptance criteria |
By the personnel responsible for quality | |
| 237 |
10.2.2
|
Define the method of reprocessing | And approve the method | |
| 238 |
10.2.3
|
Perform controls on the reprocessed finished products | And also on the reprocessed bulk products | |
|
239
|
10.2.3
|
Review results by authorized personnel |
In order to verify the conformity of the finished product or bulk product with the acceptance criteria |
|
|
11
|
Wastes
|
|||
| 11.1 |
Principle
|
|
||
| 240 |
11.1
|
Dispose of wastes in a timely manner | And also in a sanitary manner | |
|
|
11.2 |
Types of waste
|
||
| 241 |
11.2
|
Define the different types of waste from production and the laboratory |
Waste that could affect the quality of the product | |
| 11.3 |
Flow
|
|
||
|
242
|
11.3.1
|
Ensure that the flow of waste does not affect production operations |
And laboratory operations | |
|
243
|
11.3.2
|
Take appropriate measures concerning wastes |
Including collection, storage and disposal of | |
|
|
11.4 |
Containers
|
|
|
| 244 |
11.4
|
Identify properly containers of waste | As to contents and other information, as appropriate | |
| 11.5 |
Disposal
|
|
||
| 245 |
11.5
|
Perform the disposal of waste in an appropriate way | With an adequate level of control | |
|
Subcontracting
|
||||
|
|
Principle
|
|
||
| 246 | 12.1 |
Establish a contract or agreement for subcontracted activities |
The contract or agreement being mutually confirmed and controlled (between the contract giver and the contract acceptor) |
|
| 247 | 12.1 |
Obtain a product or service that complies with the requirements defined by the contract giver |
This is the objective of this step | |
|
|
12.2 |
Types of subcontracting
|
||
| 248 |
12.2 a
|
Take into account subcontracting such as |
Manufacturing | |
| 249 |
12.2 b
|
Take into account subcontracting such as | Packaging | |
| 250 |
12.2 c
|
Take into account subcontracting such as | Analysis | |
| 251 |
12.2 d
|
Take into account subcontracting such as | Cleaning, sanitization of premises | |
| 252 |
12.2 e
|
Take into account subcontracting such as | Pest control | |
| 253 |
12.2 f
|
Take into account subcontracting such as | Equipment and premises maintenance | |
|
|
Contract giver
|
|
||
|
254
|
12.3.1
|
Assess the ability and capacity of the contract acceptor |
To carry out the contracted operations | |
|
255
|
12.3.1
|
Ensure that the contract acceptor has all the necessary means at its disposal |
To carry out the contract | |
|
256
|
12.3.1
|
Assess the subcontractor's ability to comply appropriately |
With ISO 22716 guidelines | |
| 257 |
12.3.1
|
Assess the subcontractor's ability to ensure the operations can be performed |
As agreed. The contract acceptor shall comply with the requirements of cosmetic Good Manufacturing Practices that apply to it |
|
|
258
|
12.3.2
|
Provide the contract acceptor with all the information required | In order to carry out the operations correctly | |
|
|
12.4 |
Contract acceptor
|
||
| 259 |
12.4.1
|
Ensure that the contract acceptor has the means, experience and competent personnel |
In order to meet the requirements of the contract | |
|
260
|
12.4.2
|
Ensure that the contract acceptor does not subcontract work to a third party |
Without the prior agreement and consent of the contract giver |
|
| 261 |
12.4.2
|
Make arrangements between the third party and the contract acceptor |
In order to ensure that all information about operations is made available to the contract giver in the same way as in the original contract |
|
|
262
|
12.4.3
|
Facilitate any checks and audits defined by the contract giver |
Activities at the contract acceptor | |
|
263
|
12.4.4
|
Inform the contract giver of any changes prior to implementation, unless otherwise specified in the contract |
Any changes that may affect the quality of the services or products provided |
|
|
|
Contract
|
|
||
| 264 |
12.5.1
|
Draw up a contract or agreement specifying respective duties and responsibilities |
Between the contract giver and the contract acceptor |
|
| 265 |
12.5.2
|
Keep all data |
Or they shall be made available to the contract giver |
|
|
Deviations
|
||||
| 266 | 13.1 |
Authorize deviations from the specified requirements (waivers) |
On the basis of sufficient data to justify the decision. Analyze and evaluate the consequences. Control changes, cf. clause 15 |
|
| 267 | 13.2 | Make corrective actions |
In order to prevent recurrence of the deviation |
|
| 14 |
Complaints and recalls
|
|||
|
|
Principle
|
|||
| 268 |
14.1.1
|
Review, investigate and follow up, as appropriate, all complaints that are communicated to the plant |
Complaints fall within the scope of ISO 22716 guidelines |
|
| 269 |
14.1.2
|
Take appropriate steps when a product recall decision is made |
In order to carry out the recall within the scope of the ISO 22716 guidelines |
|
| 270 |
14.1.2
|
Take appropriate steps when a product recall decision is made | And to implement corrective action | |
| 271 |
14.1.3
|
Agree on how to handle complaints |
In the case of contracted operations, cf. § 12 | |
|
|
Product complaints
|
|
||
|
272
|
Centralize all complaints | By authorized personnel | ||
|
273
|
14.2.2
|
Keep any complaints concerning a product defect |
Including original details and follow-up information, cf. clause 17 | |
| 274 | 14.2.3 |
Complete an appropriate follow-up of the batch concerned |
To the end of its term | |
| 275 |
14.2.4 a
|
Include in complaints investigations and follow-up |
Steps to prevent recurrence of the defect |
|
|
276
|
14.2.4 b
|
Include in complaints investigations and follow-up | checking other batches in order to determine whether they are also affected | |
| 277 |
14.2.5
|
Review periodically complaints |
In order to check for trends or recurrence of a defect |
|
| 14.3 |
Product recalls
|
|||
|
278
|
14.3.1
|
Coordinate the recall process | By authorized personnel | |
| 279 |
14.3.2
|
Initiate product recall operations | Promptly and in a timely manner | |
|
280
|
14.3.3
|
Notify the competent authorities of any recall |
Which could have an impact upon consumer safety |
|
|
281
|
14.3.4
|
Identify and store the recalled products separately in a secure area |
While awaiting a decision. The secure area is "the prison", area with restricted access |
|
| 282 |
14.3.5
|
Evaluate periodically the product recall process | Cf. § 14.1.2. This process is most often a simulation test. | |
| 15 |
Change control
|
|||
| 283 |
15
|
Approve and perform changes that may affect the quality of the product (product evolution) |
By authorized personnel on the basis of sufficient data | |
|
Internal audit
|
||||
|
|
Principle
|
|||
| 284 | 16.1 |
Monitor the implementation and the status of cosmetic Good Manufacturing Practices and, where appropriate, propose corrective actions |
This is the main objective of the internal audit | |
|
|
16.2 |
Approach
|
|
|
| 285 |
16.2.1
|
Conduct independent and detailed internal audits, on a regular basis or upon request |
By competent, specially designated personnel | |
| 286 |
16.2.2
|
Evaluate all observations made during the internal audit | And shared with appropriate management | |
|
|
Follow-up
|
|
||
| 287 |
16.3
|
Confirm the satisfactory completion or implementation of corrective action |
By audit follow-up | |
|
17
|
Documentation
|
|||
| 17.1 |
Principle
|
|||
| 288 |
17.1.1
|
Establish, design, install and maintain its own documentation system |
For each company, the system is appropriate to its organizational structure and product type. The system can be in paper or electronic format |
|
|
289
|
17.1.2
|
Integrate documentation into Good Manufacturing Practices |
There's no way around it. | |
| 290 |
17.1.2
|
Describe activities defined in the ISO 22716 guidelines in order to relate the history of these activities |
In order to avoid any risk of interpretation, loss of information, confusion or errors inherent to verbal communication |
|
| 17.2 |
Type of document
|
|
||
|
291
|
17.2.1
|
Include documents such as procedures, instructions, specifications, protocols, reports, methods and records |
All relevant to the activities of the ISO 22716 guidelines |
|
| 292 |
17.2.2
|
Choose between paper or electronic format | Or a mix of the two formats | |
|
|
17.3 |
Writing, approval and distribution
|
||
| 293 |
17.3.1
|
Define documents with appropriate detail in relation to ISO 22716 guidelines |
Describing the operations to be carried out, precautions to be taken and measures to be applied in all cosmetic activities |
|
| 294 |
17.3.2
|
State the title, nature and purpose of the documents |
Including a code | |
| 295 | 17.3.3 a | Write documents that are | Written in a legible and comprehensive way | |
| 296 | 17.3.3 b | Write documents that are | Approved, signed and dated by authorized persons before being used | |
| 297 |
17.3.3 c
|
Write documents that are | Prepared, updated, withdrawn, distributed, classified | |
| 298 |
17.3.3 d
|
Write documents that are | Referenced to ensure that obsolete documents are not used | |
| 299 |
17.3.3 e
|
Write documents that are | Accessible to appropriate personnel | |
| 300 |
17.3.3 f
|
Write documents that are | Removed from the job area and destroyed if they are out-dated | |
| 301 |
17.3.4 a
|
Indicate what is to be entered | For records that require data entry (handwritten or electronic) | |
| 302 |
17.3.4 b
|
Write legibly with permanent ink | For records that require data entry (handwritten or electronic) | |
| 303 |
17.3.4 c
|
Sign and date | For records that require data entry (handwritten or electronic) | |
| 304 |
17.3.4 d
|
Correct, if necessary, by allowing the original text to be read; where appropriate, record the reason for the correction |
For records that require data entry (handwritten or electronic) | |
|
|
Revision
|
|||
|
305
|
17.4
|
Update documents when necessary | And indicate the revision number | |
|
306
|
17.4
|
Retain the reason for each revision | On the document or on an archive file | |
| 17.5 |
Archiving
|
|
||
| 307 |
17.5.1
|
Archive only original documents | And use only controlled copies | |
|
308
|
17.5.2
|
Define the duration of archiving original documents | According to applicable laws and regulations | |
| 309 |
17.5.3
|
Secure properly the storage of original documents | Appropriately | |
|
310
|
17.5.4
|
Ensure the legibility of archived documents | In paper or electronic format | |
| 311 | 17.5.5 | Save data at regular intervals | In separate and secure location | |
|
|
|
|
||




